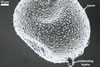
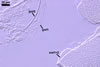
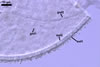
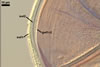
REFERENCES
Blaszkowski
J. 1988a. Four new species of the Endogonaceae
(Zygomycotina) from Poland. Karstenia 27, 37-42.
Blaszkowski
J. 1988b. Three new vesicular-arbuscular mycorrhizal
fungi (Endogonaceae) from Poland. Bull. Pol.
Ac. Sci. Biol. Sci. 36, 10-12.
Blaszkowski
J. 1993. Comparative studies of the occurrence
of arbuscular fungi and mycorrhizae (Glomales)
in cultivated and uncultivated soils of Poland.
Acta Mycol. 28, 93-140.
Blaszkowski
J. 1994. Arbuscular fungi and mycorrhizae (Glomales)
of the Hel Peninsula, Poland. Mycorrhiza 5,
71-88.
Blaszkowski
J., Tadych M., Madej T., Adamska I., Iwaniuk
A. 2001. Arbuscular mycorrhizal fungi (Glomales,
Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej
Konf. Nauk. nt. „Gospodarowanie zasobami
wodnymi i nawadnianie roslin uprawnych”.
Przeglad naukowy Wydz. Inz. Ksztalt. Srod. 22,
8-27.
Kennedy
L. J., Stutz J. C., Morton J. B. 1999. Glomus
eburneum and G. luteum, two new
species of arbuscular mycorrhizal fungi, with
emendation of G. spurcum. Mycologia
91, 1083-1093.
Morton
J. B., Redecker D. 2001. Two families of Glomales,
Archaeosporaceae and Paraglomaceae, with two
new genera Archaeospora and Paraglomus,
based on concordant molecular and morphological
characters. Mycologia 93, 181-195.
Morton
J. B., Walker C. 1984. Glomus diaphanum:
a new species in the Endogonaceae common to
West Virginia. Mycotaxon 21, 431- 440.
Tadych
M., Blaszkowski J. 2000. Arbuscular fungi and
mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.
Walker
C. 1982. Species in the Endogonaceae: a new
species (Glomus occultum) and a new
combination (Glomus geosporum). Mycotaxon
15, 49-61.
Walker
C., Giovannetti M., Avio L., Citernesi A. S.,
Nicolson T. H. 1995. A new fungal species forming
arbuscular mycorrhizas: Glomus viscosum.
Mycol. Res. 99, 1500-1506.
Wu
C.-G., Liu Y.-S., Hwuang Y.-L., Wang Y.-P.,
Chao C.-C. 1995. Glomales of Taiwan: V. Glomus
chimonobambusae and Entrophospora kentinensis,
spp. nov. Mycotaxon 53, 283-294.