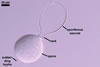
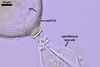
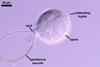
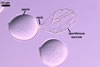
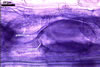
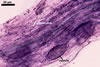
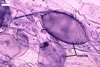
GERMINATION.
Not observed.
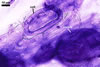
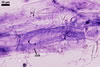
MYCORRHIZAE. In roots of Plantago lanceolata L., I. schenckii formed arbuscules, vesicles, and intraradical hyphae with coils. All the structures stained intensively in 0.1% trypan blue.
|
|
|
|
|
|
|
|
|
In roots of P.
lanceolata |
DISTRIBUTION.
In
Poland, spores of I. schenckii have been found only in one
trap culture with a soil and root mixture taken from under Juniperus
communis L. growing in the Bledowska Desert (50º22’N, 19º34’E).
The
type of I. schenckii has been isolated from a pot culture of
tropical kudzu established with soil coming from a rose nursery located
in Melecio Ospina, Colombia, South America (Sieverding and Toro 1987).
Other known sites of the occurrence of this fungus are those located in Southern Brazil (Sieverding and Oehl 2006), Argentina (Fracchia et al. 2003), Swiss Alp (Sieverding and Oehl 2006), India (Mohankumar et al. 1988; Ragupathy and Mahadevan 1993), and in Thailand (Bhadalung et al. 2005).
NOTES. According to Sieverding and Oehl (2006), the inner germination wall of spores of I. schenckii consists of three layers,
and not of one layer as described above.
The layer characterized above is located in the middle and is surrounded by two thin, hyaline layers. All these layers are tightly adherent to and rarely separate from each other and, thereby, are exceptionally difficult to observe.
When spores of I. schenckii lack the sporiferous saccule, they
are almost indistinguishable from those of Archaeospora
trappei. Spores of the two fungi are
colourless and overlap in size range. Moreover, the spore wall structure
of the
two
species is identical in both the number of layers and their phenotypic
properties. However, the pear-shaped spores with the swellings formed in the region of their contact with the neck of the sporiferous saccule clearly suggest their affinity to I. schenckii.
Intraspora schenckii has originally been described as Entrophospora schenckii (Sieverding and Toro 1987). The main arguments used to exclude this fungus from the genus Entrophospora were (1) the simple structure of its spore wall relative to E. infrequens, the type species of this genus, and E. baltica, the only other species remained in the revised genus Entrophospora (Sieverding and Oehl 2006), (2) the formation of spores at some distance from the sporiferous saccule, and not directly at it as in Entrophospora spp., and (3) the faint staining of mycorrhizal structures of I. schenckii in contrast to the intensively staining mycorrhizae of E. infrequens, the only species of Entrophospora propagated in one-species cultures (Blaszkowski et al. 1998; Sieverding and Oehl 2006;
Sieverding and Toro 1986).
REFERENCES
Bhadalung N. N., Suwanarit A., Dell B., Nopamornbodi O., Thamchaipenet A., Ruangchuang J. 2005. Effects of long-term NP-fertilization on abundance and diversity of arbuscular mycorrhizal fungi under a maize cropping system. Plant and Soil 270, 371-382.
Blaszkowski J., Madej T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus fuegianum, two species in the Glomales from Poland. Mycotaxon 68, 165-184.
Fracchia S., Scervino J. M., Menendez A., Godeas A. M. 2003. Isolation, culture and host colonization of Entrophospora schenckii (Glomales), an arbuscular mycorrhizal fungus. Nova Hedwigia 77, 383-388.
Mohankumar
V., Ragupathy S., Nirmala C. B., Mohadevan A. 1988. Distribution of
vesicular-arbuscular mycorrhizae (VAM) in the sandy beach soils of Madras
coast. Cur. Sci. 57, 367-368.
Ragupathy S., Mahadevan A. 1993. Distribution of vesicular-arbuscular mycorrhizae in the plants and rhizosphere soils of the tropical plains, Tamil Nadu, India. Mycorrhiza 3, 123-136.
Sieverding E., Oehl F. 2006. Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. J. Appl. Bot. Food Qual. 80, 69-81.
Sieverding E., S. Toro T. 1986. The genus Entrophospora in Colombia. In: Gianinazzi-Pearson V., Gianinazzi S. (eds). Physiological and genetical aspects of mycorrhizae. Proc. 1st European Symposioum on Mycorrhizae. Dijon, 1-5 July 1985, 621-626.
Sieverding
E., Toro S. 1987. Entrophospora schenckii: a new species in
the Endogonaceae from Colombia. Mycotaxon 28, 209-214.