

F.M. Rothwell & Trappe
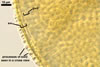
SPORES single in the soil; develop laterally on the neck of a sporiferous saccule; sessile; light orange (5A5) to yellowish brown (5E8); globose to subglobose; (150-)190(-210) µm diam; sometimes irregular; 130-180 x 170-250 µm.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and two inner germination walls.
 |
 |
In PVLG |
|
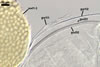
Layer 1, forming the spore surafce, evanescent, light yellow (4A4) to apricot yellow (5B6), (0.7-) 1.1(-2.0) µm thick, closely attached to wall 2, continuous with the wall of a sporiferous saccule, usually completely sloughed in mature spores.
Layer 2 laminate, ornamented, light orange (5A5) to yellowish brown (5E8), (6.8-)7.1(-7.4) µm thick; ornamentation consists of hyaline to yellowish white (3A2) spines, (0.7-)1.0(-1.7) µm high and of ridges forming a quadrilateral to heptagonal reticulum when seen in a plan view; ridges hyaline to yellowish white (3A2), (2.2-) 3.7 (-4.4) µm high, (1.0-)1.6(-2.5) µm wide at the base, (1.2-)2.7(-4.4) µm wide at the top, (1.2-)3.9(-6.6) µm high when seen in a cross view.
Layer 3 laminate, hyaline, (0.8-)1.2(-1.6) µm thick, usually tightly adherent to layer 2.
 |
 |
 |
 |
 |
 |
 |
In PVLG |
||||||
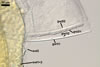
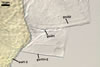
Germination wall 1 composed of two semirigid, hyaline layers (gw1l1 and 2).
Layer 1 (0.4-) 0.5 (-0.7) µm thick, rarely separating from layer 2.
Layer 2 (1.0-) 1.4 (-1.5) µm thick.
Germination wall 2 consists of two layers (gw2l1 and 2).
Layer 1 flexible, hyaline, ca. 0.5 µm thick, covered with small granules.
Layer 2 (3.4-)4.5(-5.9) µm thick, tightly adherent to layer 2, staining pale red (7A3) in Melzer's reagent.
GERMINATION ORB. Not found.
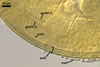
SPORIFEROUS SACCULE light yellow (4A4) to apricot yellow (5B6); globose to subglobose; 200-250 µm diam; neck 120-200 µm long, tapering from 45-50 µm diam at the saccule to 20-30 µm diam at the point of spore attachment. Saccule wall of a light yellow (4A4) to apricot yellow (5B6), smooth, 1.7-2.8 µm thick layer. Saccule collapsing at maturity and usually becoming detached in mature spores.
 |
In PVLG |
CICATRIX. A small scar, 7.6-10.0 µm diam, surrounding a pore, 1.0-1.2 µm diam present at the site of spore attachment to the saccule.
GERMINATION. Unknown.
MYCORRHIZAE. In Poland, Acaulospora bireticulata has been associated in the field with vesicular-arbuscular mycorrhizae of Ammophila arenaria (L.) Link, Artemisia campestris L., Juniperus communis L., Triticum aestivum L., and an unknown grass (Blaszkowski 1989, 1997; Tadych and Blaszkowski 2000). Attempts to establish this species in both trap and one-species pot cultures with Sorghum sudanense (Staph.) Piper failed. No literature data exist of the properties of mycorrhizae of Ac. bireticulata coming from its one-species cultures.
DISTRIBUTION. In Poland, spores of A. bireticulata have been found in the root zone of Ammophila arenaria and Artemisia campestris colonizing maritime sand dune soils adjacent to Swinoujscie (53º55'N, 14º14'E), Triticum aestivum, cultivated in the Western Pomerania, J. communis growing in the Tuchola Forests (53º46’N, 17º42’E-53º40’N, 17º54’E), and among roots of an unknown grass (Blaszkowski 1989, 1997; Tadych and Blaszkowski 2000).
Although A. bireticulata probably has a worldwide distribution, this fungus occurs rarely. Rothwell and Trappe (1979) originally recovered spores of this species from a perimeter soil sample collected under Sassafrans albidum (Nutt.) Ness growing in western Kentucky and from a greenhouse culture with Zea mays L. Schenck and Smith (1981) found A. bireticulata spores in association with Centrosema pubescens L. in Florida. Miller et al. (1985) isolated this fungus from the root zone of Malus domestica Borkh. in Michigan. Walker (pers. comm.) recorded this species in sand dunes in the United Kingdom.
NOTES. The most distinctive morphological character of spores of A. birticulata is the ornamentation of the upper surface of the spore wall layer 2 consisting of a polygonal reticulum overlaying crowded spines. The light orange to yellowish brown spore colour is formed by pigment mostly concentrated in its relatively thin outer layer.
When observed under a dissecting microscope, spores of A. bireticulata may resemble those of A. cavernata, A. denticulata, A. excavata, A. rehmii, A. spinosa, and A. tuberculata due to their roughened surface and similarities in colour and size. Examination of crushed spores under a light microscope readily distinguishes A. bireticulata from all the species listed above having a structural wall ornamented with tooth-shaped projections (A. denticulata; Blaszkowski 2003; Sieverding and Toro 1987; Morton 2002), pits (A. excavata, A. foveata, A. cavernata; Baszkowski 1989, 2003; Ingleby et al. 1994; Janos and Trappe 1982), labyrinthiform folds (A. rehmii; Blaszkowski 2003; Sieverding and Toro 1987), crowded blunt spines (A. spinosa; Morton 2002; Walker and Trappe 1981), and tubercles (A. tuberculata; Janos and Trappe 1982; Morton 2002). Acaulospora elegans is described to possess spores ornamented with spines and an alveolate reticulum (Gerdemann and Trappe 1974). According to Morton (2002) and Walker (pers. comm.), A. elegans and A. bireticulata probably are congeneric. However, no formal synonymization of A. bireticulata with A. elegans has so far been made.
REFERENCES
Blaszkowski J. 1989. Polish Endogonaceae 1. Acaulospora bireticulata, Entrophospora infrequens, Glomus caledonium, and Scutellospora pellucida. Karstenia 29, 1-10.
Blaszkowski J. 1997. Notes on Acaulospora bireticulata (Glomales, Zygomycetes) found in Poland. Mycotaxon 61, 193-204.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Ingleby K., Walker C., Mason P. A. 1994. Acaulospora excavata sp. nov. - an endomycorrhizal fungus from Cote D'Ivoire. Mycotaxon 50, 99-105.
Janos D. P., Trappe J. M. 1982. Two new Acaulospora species from tropical America. Mycotaxon 15, 515-522.
Miller D. D., Domoto P. A., Walker C. 1985. Mycorrhizal fungi at eighteen apple rootstock plantings in the United States. New Phytol. 100, 379-391.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Rothwell F. M., Trappe J. M. 1979. Acaulospora bireticulata sp. nov. Mycotaxon 8, 471-475.
Schenck N. C., Smith G. S. 1981. Distribution and occurrence of vesicular-arbuscular mycorrhizal fungi on Florida agricultural crops. Soil Crop Sci. Florida, Proc. 40, 171-175.
Sieverding E., Toro S. T. 1987. Acaulospora denticulata sp. nov. and Acaulospora rehmii sp. nov. (Endogonaceae) with ornamented spore walls. Angew. Bot. 61, 217-223.
Tadych M., Blaszkowski J. 2000. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola Forests. Acta Mycol. 35, 3-23.
Walker C., Trappe J. M. 1981. Acaulospora spinosa sp. nov. with a key to the species of Acaulospora. Mycotaxon 12, 515-521.