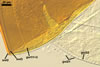





SPORES single in the soil; develop laterally on the neck of a sporiferous saccule; orange red (8A8) to capsicum red (8B8); globose to subglobose; (170-)298(-330) µm diam; sometimes irregular; 220-310 x 290-440 µm.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and two inner germination walls.
 |
 |
 |
 |
 |
 |
 |
In PVLG |
||||||
 |
 |
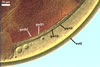 |
In PVLG+Melzer's reagent |
||
Layer 1, forming the spore surface, evanescent, hyaline, (0.8-)1.5(-2.5) µm thick, continuous with the wall of a sporiferous saccule, usually completely sloughed in mature spores.
Layer 2 laminate, smooth, orange red (8A8) to capsicum red (8B8), (2.2-)4.2(-5.6) µm thick.
Layer 3 laminate, rigid, hyaline, (1.2-)2.4(-4.6) µm thick, easily separating from layer 2.
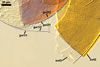
Germination wall 1 composed of two hyaline layers (gw1l1 and 2).
Layer 1 flexible, ca. 0.5 µm thick, rarely separating from layer 2.
Layer 2 flexible to semi-flexible, 1.2-3.2 µm thick.
Germination wall 2 consists of two layers (gw2l1 and 2).
Layer 1 flexible, hyaline, covered with small granules, (0.7-)1.4(-2.7) µm thick.
Layer 2 plastic, 2-20 µm thick in PVLG, 1.2-1.8 µm thick and deep magenta (13E8) in Melzer’s reagent.
GERMINATION ORB. Not found.
 |
In PVLG |
SPORIFEROUS SACCULE pale yellow (3A3) to brownish yellow (5C8); globose to subglobose; 180-360 µm diam; neck 130-150 µm long, 40-50 µm wide at the saccule, tapering to 28-40 µm wide at the spore attachment. Saccule usually collapses or falls off in mature spores.
In PVLG |
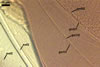
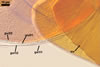
CICATRIX. A slightly raised collar, 1.5-3.0 µm wide x 1.0-2.5 µm long, surrounding a hole, 13-20 µm diam.
MYCORRHIZAE. In the field, Ac. capsicula has been associated with vesicular-arbuscular mycorrhizal roots of Ficaria verna Huds., Juncus conglomeratus L., Salix triandra L., Thuja occidentalis L., and Triticum aestivum L. (Błaszkowski 1990). In one-species cultures with Trifolium repens L. as the plant host, this fungus formed mycorrhizae with arbuscules, vesicles, as well as with intra- and extraradical hyphae staining intensively in 0.05% trypan blue.
DISTRIBUTION. Acaulospora capsicula has originally been characterized from spores isolated from among roots of F. verna growing in a forest of Zelistrzewo located in northern Poland (54º41’N, 18º27’E; Błaszkowski 1990). Other Polish collections of this fungus are those from under S. triandra and T. occidentalis cultivated in Hel (54º36’N, 18º49’E; Błaszkowski 1990, 1994), J. conglomeratus growing in a wet meadow in Oslonino (54º42’N, 18º28’E; Błaszkowski 1990, 1993), and Trit. aestivum cultivated in Wardyn (53º10’N, 15º30’E) located in the Western Pomerania (Iwaniuk and Błaszkowski 2004a, b). Also found in North Carolina, Durham County, in soils of an old field with a diverse plant community placed on Duke University Campus (treated as A. collosica; Schultz et al. 1999).
There is no report of the presence of A. capsicula in other regions of the world.
NOTES. Spores of A. capsicula resemble those of A. koskei and A. laevis. The unique property of the former species is the capsicum red colour of its spores. Although the subcellular structure of spores of A. capsicula and A. laevis is identical, all layers of the spore wall and the germination walls in A. capsicula are thicker (Morton 2002). The most important property separating A. capsicula and A. koskei is their third layer of the spore wall. In the latter species, it is thinner and stains in Melzer’s reagent (Błaszkowski 1995; vs. it is nonreactive in A. capsicula).
Acaulospora colossica is a synonym of A. capsicula (Morton 2002).
REFERENCES
Błaszkowski J. 1990. Polish Endogonaceae. VII. Acaulospora capsicula sp. nov. Mycologia 82, 794-798.
Błaszkowski J. 1993. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Błaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Błaszkowski J. 1995. Acaulospora koskei, a new species in Glomales from Poland. Mycol. Res. 99, 237-240.
Iwaniuk A., Błaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part I. Occurrence of arbuscular fungi and mycorrhizae. Acta Mycol. 39(1), 59-84.
Iwaniuk A., Błaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part II. Distribution of arbuscular fungi. Acta Mycol. 39(2), 3-18.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Schultz P. A., Bever J. D., Morton J. B. 1999. Acaulospora colossica sp. nov. from an old field in North Carolina and morphological comparisons with similar species, A. laevis and A. koskei. Mycologia 91, 676-683.