 |
||
In PVLG |
In PVLG+Melzer's reagent |
|
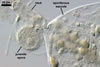
SPORES single in the soil; develop laterally on the neck of a sporiferous saccule; hyaline to pale yellow (3A3); globose to subglobose; (60-)72(-95) µm diam; sometimes ovoid; 60-70 x 80-95 µm.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and two inner germination walls.
|
In PVLG |
|||||
In PVLG |
In PVLG+Melzer's reagent
|
||||
Spore wall composed of three layers (swl1-3).
Layer 1 evanescent, hyaline, 0.5-0.8 µm thick, continuous with the wall of a sporiferous saccule, usually completely sloughed in mature spores.
Layer 2 laminate, hyaline to pale yellow (3A3), (1.5-)2.0(-2.5) µm thick, ornamented with evenly distributed pits, 2.0-2.5 x 3.0-3.5 µm, when seen in a plan view, 0.8-1.0 µm deep, when observed in a cross-sectional view.
Layer 3 flexible, hyaline, <0.5 µm thick, tightly adherent to layer 2, separates from layer 2 usually only in vigorously crushed spores.
Germination wall 1 consists of two hyaline, flexible to semiflexible layers (gw1l1 and 2), each ca. 0.5 µm thick; these layers usually separate from each other in crushed spores.
Germination wall 2 composed of two adherent layers (gw2l1 and 2).
Layer 1 flexible, hyaline, covered with small, <0.5 µm diam, granules sometimes scattering in crushed spores.
Layer 2 plastic, hyaline, 10-15 µm thick in PVLG, (0.5-)0.8(-1.0) µm thick and pale red (12A3) in Melzer’s reagent.
GERMINATION ORB. Not found.
SPORIFEROUS SACCULE hyaline; globose to subglobose; 60-85 µm diam; neck 70-95 µm long, 10.0-16.5 µm wide at the saccule, tapering to 6.5-8.0 µm wide at the spore attachment. Saccule usually collapses or falls off in mature spores.
 |
 |
In PVLG |
|
CICATRIX. A slightly raised collar when seen in a cross view, circular, 6.0-9.0 µm diam, when observed in a plane view.
MYCORRHIZAE. Acaulospora paulinae has co-occurred with vesicular-arbuscular mycorrhizal roots of many plant species (Błaszkowski 1993a, b, 1994; Błaszkowski et al. 2001; Tadych and Błaszkowski 2000a, b; Iwaniuk and Błaszkowski 2004). However, the characters of mycorrhizae of this fungus from its one-species cultures have not been determined yet.
DISTRIBUTION. In Poland, Ac. paulinae has been recorded in dunes of the Gdansk coast, the Hel Peninsula (54º47’N, 18º25’E-54º36’N, 18º49’E), and the Slowinski National Park (54º45’N, 17º26’E; Błaszkowski 1993a, 1994; Tadych and Błaszkowski 2000a), in soils of the Tuchola Forests (53º46’N, 17º42’E-53º40’N, 17º54’E; Tadych and Błaszkowski 2000b), as well as in cultivated sites of the Western Pomerania and the Pomerania voivodeships (Błaszkowski 1993b; Iwaniuk and Błaszkowski 2004).
Koske et al. (1997) encountered this fungus associated with Agrostis canina Huds., A. palustris L., and Poa annua L., perennial turf species of golf greens of Rhode Island, U. S. A. Additionally, Ac. paulinae has been recognized in dunes adjacent to Tel-Aviv (32º4’N, 34º46’E), Israel (Błaszkowski et al. 2001).
NOTES. Apart from Ac. paulinae, other species of the genus Acaulospora forming pitted spores are Ac. alpina Oehl et al., Ac. cavernata Blaszk., Ac. excavata Ingleby & C. Walker, Ac. foveata Trappe & Janos, Ac. lacunosa J.B. Morton, Ac. scrobiculata Trappe, Ac. taiwania H.T. Hu, and Ac. undulata Sieverd.
When observed under a dissecting microscope, the fungus producing spores most similar in size and colour to those of Ac. paulinae is Ac. undulata. Examination of spores of these fungi under a compound microscope readily separate them. First, the pits ornamenting the spore wall layer 2 of Ac. paulinae are ca. 2-3-fold smaller in diameter when circular than the circular pits of the laminate spore wall layer of Ac. undulata (Błaszkowski 1988; Sieverding 1988). Second, the proportion of irregular pits in the total number of pits in spores of the former fungus is markedly lower than in those of the latter species. Third, Ac. paulinae has a 3-layered spore wall and two 2-layered germination walls with the innermost layer of the second wall staining red in Melzer's reagent, whereas the subcellular structure of spores of Ac. undulata consists of only a 2-layered spore wall and one 1-layered germination wall staining yellowish orange in this reagent (Sieverding 1988). Thus, spores of Ac. undulata lack the second inner germination wall of Ac. paulinae.
Spores of Ac. alpina and Ac. taiwania overlap in size with those of Ac. paulinae, but spores of the former two species are darker-coloured (up to orange-brown vs. hyaline to pale yellow in Ac. paulinae; Hu 1988; Oehl et al. 2006). Additionally, these species differ in morphology and distribution of the pits of their laminate structural spore wall layer, as well as in the phenotypic and biochemical properties of the components of the innermost germination wall. In Ac. paulinae, the ornamentation of the laminate layer consists of concave pits or depressions, whereas truncated conic depressions and 4-5-sided pits giving the appearance of a mesh ornament the laminate spore wall layer of Ac. alpina (Oehl et al. 2006) and Ac. taiwania (Hu 1988), respectively. The outer layer of the second germination wall of spores of Ac. paulinae is covered with small granules, i.e., it is “beaded”, and adheres to a plastic layer staining intensively in Melzer's reagent. In contrast, the outer layer of the second germination wall of spores of Ac. alpina is smooth, and the second layer of this wall stains weakly or does not stain at all in Melzer's reagent. Still another difference between the species compared here is that spores of Ac. taiwania occur in sporocarps (Hu 1988), and Ac. alpina and Ac. paulinae form only single spores. Finally, results of molecular studies showed no relationship between Ac. paulinae and Ac. alpina (Oehl et al. 2006).
In contrast, Ac. scrobiculata produces light-coloured spores as those of Ac. paulinae. However, spores of Ac. scobiculata are much larger (Błaszkowski 2003; Morton 2002; Trappe 1977) than those of Ac. paulinae.
Spores of Ac. cavernata, Ac. excavata, Ac. foveata, and Ac. lacunosa are much darker and larger (Błaszkowski 1989, 1990; Ingleby et al. 1994; Janos and Trappe 1982; Morton 1986, 2002). Additionally, the pits in the spore wall layer 2 of Ac. lacunosa are more irregular in shape and distribution (Błaszkowski 2003; Morton 2002).
Results of molecular analyses of spores of Ac. paulinae located this fungus in a distinct clade sister to Ac. longula Spain & N.C. Schenck (=Ac. morrowiae Spain & N.C. Schenck according to Morton 2002) within the family Acaulosporaceae J.B. Morton & Benny in the order Diversisporales C. Walker & Schuessler (Gamber and Leuchtmann 2007).
REFERENCES
Błaszkowski J. 1988. Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland. Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Błaszkowski J. 1989. Acaulospora cavernata (Endogonaceae) - a new species from Poland with pitted spores. Crypt. Bot. 1, 204-207.
Błaszkowski J. 1990. Polish Endogonaceae. VI. Acaulospora lacunosa. Crypt. Bot. 2, 20-24.
Błaszkowski J. 1993a. The occurrence of arbuscular fungi and mycorrhizae (Glomales) in plant communities of maritime dunes and shores of Poland. Bull. Pol. Ac. Sci. Biol. Sci. 41, 377-392.
Błaszkowski J. 1993b. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Błaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland.http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Błaszkowski J., Tadych M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz. Inz. Ksztalt. Srod. 22, 8-27.
Gamber H., Leuchtmann A. 2007. Taxon-specific PCR primers to detect two inconspicuous arbuscular mycorrhizal fungi from temperate agricultural grassland. Mycorrhiza 17, 145-152.
Hu H. T. 1988. Study on the endomycorrhizae of China fir (Cunninghamia lanceolata Hooker) and taiwania (Taiwania cryptomerioides). Q. J. Chin. For. 21, 45-72.
Ingleby K., Walker C., Mason P. A. 1994. Acaulospora excavata sp. nov. - an endomycorrhizal fungus from Cote D'Ivoire. Mycotaxon 50, 99-105.
Iwaniuk A., Błaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part I. Occurrence of arbuscular fungi and mycorrhizae. Acta Mycol. 39(1), 59-84.
Janos D. P., Trappe J. M. 1982. Two new Acaulospora species from tropical America. Mycotaxon 15, 515-522.
Koske R. E, Gemma J. N, Jackson N. 1997. Mycorrhizal fungi associated with three species of turfgrass. Can. J. Bot. 75, 320-332.
Morton J. B. 1986. Three new species of Acaulospora (Endogonaceae) from high aluminum, low pH soils in West Virginia. Mycologia 78, 641-648.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Oehl F., Sýkorová Z., Redecker D., Wiemken A. 2006. Acaulospora alpina, a new arbuscular mycorrhizal fungal species characteristic for high mountainous and alpine regions of the Swiss Alps. Mycologia 98, 286-294.
Sieverding E. 1988. Two new species of vesicular arbuscular mycorrhizal fungi in the Endogonaceae from tropical high lands of Africa. Angew. Bot. 62, 373-380.
Tadych M., Błaszkowski J. 2000a. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National Park, Poland. Mycotaxon 74, 463-483.
Tadych M., Błaszkowski J. 2000b. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola Forests. Acta Mycol. 35, 3-23.
Trappe J. W. 1977. Three new Endogonaceae: Glomus constrictus, Sclerocystis clavispora, and Acaulospora scrobiculata. Mycotaxon 6, 359-366.