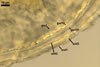
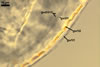
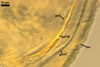
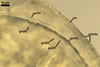
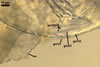
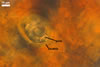
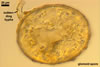
Appendicispora appendicula
(Spain, Sieverd. & N.C. Schenck) Spain, Oehl & Sieverd.
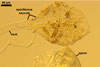
A dimorphic fungus, producing acaulosporioid and glomoid spores. Acaulosporioid spores formed singly, and glomoid ones either singly or in loose aggregates in the soil.
ACAULOSPORIOID SPORES origin blastically at the tip of a short branch (pedicel) of the neck of a sporiferous saccule continuous with a thick-walled, often rigid, persistent, 12-20(-25) µm wide mycorrhizal extraradical hypha. Spores pale yellow (4A3) to butter yellow (4A5); globose to subglobose; (170-)250(-390) µm diam. .............
SUBCELLULAR
STRUCTURE OF ACAULOSPORIOID SPORES consists of a spore wall and two inner germination walls.
Spore wall composed of three layers
(swl1-3).
|
|
|
|
|
|
|
|
|
In PVLG |
In PVLG+Melzer's reagent |
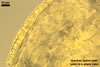
Layer 1, forming the spore surface, evanescent, short-lived, hyaline, 0.5-1.0 µm thick, usually highly degraded or completely sloughed in even young spores.
Layer 2 friable, pale yellow (4A3) to butter yellow (4A5), (6.9-)12.9(-21.8) µm thick, of the upper surface somewhat roughened and with an irregular crazed pattern of fine cracks, usually highly degraded in mature and older spores.
Layer 3 flexible to semi-flexible, hyaline, ca. 0.5-1.0 µm thick, always tightly adherent to the lower surface of layer 2, and, thereby, difficult to see.
Germination wall 1 comprises two semirigid to rigid, fragile, hyaline ornamented layers (gw1l1 and 2), usually separating from one another in crushed spores.
Layer 1 (2.0-)3.0(-5.7) µm thick, of the lower surface ornamented with knobby processes, 4.9-10.0 µm wide and 1.0-4.5 µm high, directed towards the spore centre.
Layer 2 (2.0-)4.9(-6.7) µm thick, of the upper surface ornamented with pits fitting in size, shape, and distribution the processes of the lower surface of layer 1. When layers 1 and 2 are adherent, the processes of layer 1 tightly fill the pits of layer 2.
Germination wall 2 hyaline, (3.2)4.7(-8.3) µm thick, probably consisting of three, tightly adherent layers: two very thin, <0.5 µm thick, layers (gw2l1 and 3) overlaying a markedly thicker, finely laminate middle layer (gw2l2). Layers 1 and 3 rarely visible in water mounted specimens, but almost imperceptible in spores crushed in PVLG (Błaszkowski, pers. observ.; Spain et al. 2006). In Melzer's reagent,
only the spore wall layer 2 stains orange (6A8) to high red (10A8), respectively.
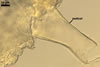
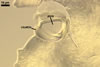
PEDICEL
hyaline to yellowish white (4A2); straight to recurved, cylindrical to slightly funnel-shaped; 30-100 µm long, 20-50 µm wide at the spore base, tapering up to 10-25 µm wide at the distal end from the spore; positioned 340-400 µm from the base of the saccule; consisting of hyaline to yellowish white (A2), 2-layered wall (phwl1 and 2) continuous with the sporiferous saccule hyphal neck, the spore wall, and layer 1 of the germination wall 1.
 |
In PVLG |
PORE
closed by a septum positioned at or up to 5 µm below the spore base, formed by layer 1 of the germination wall 1. Occasionally a second septum and less often a third one forms 20-50 µm below the spore base.
GERMINATION
ORB hyaline, subcircular, with deep incisions separating clavate lobes of an arched top when seen in a plane view, positioned between the germination walls 1 and 2 (Spain et al. 2006).
GERMINATION by a single or branched germ tube, 6-12 µm diam, emerging from the germination wall 1 or the germination orb and exiting through the pore of the pedicel (Spain et al. 2006).
SPORIFEROUS
SACCULE hyaline; globose to subglobose; (190-)250(-380) µm diam; rarely ellipsoid; 270-300 x 410-430 µm; frequently associated with young spores and usually not collapsing when detached from nature spores.
Wall of sporiferous saccule flexible to semiflexible, composed of three hyaline layers (sswl1-3): a (0.5-)1.0(-2.0) µm thick outermost layer, occasionally difficult to detect, a (2.9-)3.3(-3.7) µm thick middle layer, consisting of overlapping plate-like structures, and a thin, <0.5 µm thick, flexible innermost layer.
Saccule neck hyaline; 500-800 µm long, 45.0-57.5 µm wide at the base of the saccule, 22.5-30.0 µm wide at the spore base, then gradually tapering up to 12.5-10.0 µm wide.
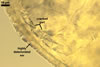
CICATRIX. A smooth pore or a slightly raised collar when seen in a cross view; circular; 27.5-35.0 µm diam; or ellipsoidal; 35.0-37.5 x 44.6-50.0 µm; when observed in a plane view.
GLOMOID SPORES
origin blastically at the tip of thin-walled hyphae, 6-12 µm diam, branched from thick-walled hyphae bearing acaulosporioid sporiferous saccules. Spores hyaline to subhyaline; globose to subglobose; (170-)187(-210) µm diam; rarely ellipsoid; 110-210 x 130-220 µm; with one subtending hypha.
 |
 |
In PVLG |
SUBCELLULAR STRUCTURE OF GLOMOID SPORES
consists of a spore wall composed of two hyaline to subhyaline layers (swl1 and 2).
Layer 1,
forming the spore surface, evanescent, (1.7-)2.5(-3.2) µm thick, frequently with adhering debris on its upper surface.
Layer 2
laminate, (4.9-)6.1(-7.3) µm thick.
SUBTENDING HYPHA
hyaline to subhyaline; straight or recurvate; cylindrical or slightly funnel-shaped; (11.0-)17.0(-24.5) µm wide at the spore base.
Wall of subtending hypha
hyaline to subhyaline; (2.2-)4.2(-5.6) µm thick at the spore base; composed of two layers continuous with spore wall layers 1 and 2.
Pore usually open, (8.8-)12.8(-18.9) µm wide, occasionally closed by a septum continuous with the inner layer of the subtending hyphal wall, positioned 5-12 µm below the spore base.
GERMINATION. By a germ tube developing from the subtending hypha (Spain et al. 2006).
MYCORRHIZAE. According to Spain et al. (2006), Ap. appendicula formed mycorrhizae with arbuscules, vesicles, and intraradical hyphae staining pale blue in trypan blue.
PHYLOGENETIC POSITION. According to Walker et al. (2007a), Ap. appendicula belongs in a monophyletic clade along with Ap. callosa (Sieverd.) C. Walker, Vestberg & Schuessler (the former Glomus callosum Sieverd.), Ap. fennica (C. Walker, Vestberg & Schuessler) C. Walker, Vestberg & Schuessler, Ap. gerdemannii (S.L. Rose, B.A. Daniels & Trappe) Spain, Oehl & Sieverd. (Archaeosporales C. Walker & Schuessler) , and Geosiphon pyriformis (Kütz.) Wettst. emend. Schüßler (Geosiphonaceae Engler. & E. Gilg emend. Schuessler) to which the clade containing Archaeospora trappei R.N. Ames & Linderman) J.B. Morton & D. Redecker emend. Spain, the type species of the genus Archaeospora J.B. Morton & D. Redecker and the family Archaeosporaceae J.B. Morton & D. Redecker emend. Oehl & Sieverd., is a sister linage.
DISTRIBUTION. The holotype of Ap. appendicula (OSC 41495) comes from spores isolated from a pot culture with Pueraria phaseoloides (Roxb.) Benth. as the host plant (culture no. C-13-1) grown at Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia (Schenck et al. 1984). This culture has originally been established from spores isolated from native grasses and tropical kutzu (P. phaseoloides ) at Carimagua, Meta province, Colombia. Schenck et al. (1984) also found spores of this fungus in acid soils (pH 5-5.5) near Gainesville and Ona, Florida, USA. Additionally, Ap. appendicula has been recorded in soils of Mexico, Brazil, Bolivia, Costa Rica, Venezuela, Switzerland, Germany, Namibia, Republic of Congo, Thailand (Spain et al. 2006), Argentina (Schalamuk et al. 2006), Great Britain (Merryweather and Fitter 1998), China (Gai et al. 2006), and Japan (Walker et al. 2007a). The only Polish finding of Ap. appendicula is that from under ....... growing ............
NOTES. The description of the morphological and biochemical properties of spores of Ap. appendicula presented above was mainly prepared based on the revised description of this fungus (Spain et al. 2006) and following examination of its specimens provided by Dr. F. Oehl, Institute of Botany, University of Basel, Switzerland; slides no. .....). Additionally, a microscope slide with well preserved spores of Ap. appendicula associated with roots of Sesleria tatrae (Degen) Deyl growing in the Mountain Botanical Garden of the Polish Academy of Sciences in Zakopane and collected by Dr. Sz. Zubek, Jagiellonian University, Kraków, Poland, was used.
Most characters of both acaulosporioid and glomoid spores of Ap. appendicula examined by the author of this website generally agreed with those of the two morphotypes given by Spain et al. (2006), who combined results of their own observations with the data of the species published by Schenck et al. (1984), as results from the two protologues. Small differences were found in (1) size of the processes of layer 1 of the germination wall 1 (4.9-10.0 µm wide x 4.5 µm high; Błaszkowski, pers. observ.; vs. 7-12 µm wide x 3-5 µm high; Spain et al. 2006), (2) thickness of layer 2 of the germination wall 1 (3.2-8.3 µm thick vs. 2-10 µm thick), (3) size of glomoid spores [170-210 µm diam vs. 120-240(-280) µm diam], (4) thickness of the spore wall layers 1 and 2 [1.7-3.2 µm thick and 4.9-7.3 µm thick, respectively vs. 1.5-2.5 µm thick and 2-8(-12) µm thick, respectively], (5) width of the subtending hypha [11.0-24.5 µm wide vs. 7-16(-19) µm wide], and (6) width of its pore [8.8-18.9 µm wide vs. 5-10(-12) µm wide].
Of the three other described species of the genus Appendicispora, Ap. appendicula is morphologically most closely related to Ap. jimgerdemannii (N.C. Schenck & T.H. Nicolson) Spain, Oehl & Sieverd. Spores of the two species are similar in appearance, colour, size, and their first inner germination wall consists of two adherent layers of an identical ornamentation (Błaszkowski, pers. observ.; Spain et al. 2006). The main differences between these species reside in the phenotypic and biochemical properties of their spore wall. Although the number and properties of the spore wall components of Ap. jimgerdemannii are not exactly known because of the poor condition of the specimens preserved and the lack of living spores of this fungus, the relatively thick (6.9-21.8 µm thick) friable spore wall layer 2 of Ap. appendicula is overlaid with a thin (0.5-1.0 µm thick) hyaline evanescent layer, whereas the second spore wall layer of Ap. jimgerdemannii is persistent, laminate, 1.0-1.5 µm thick, and covered with a thick (8-14 µm thick), brown layer of the upper surface ornamented with cerebriform folds, (6-)10-12 µm high and 4-6 µm wide spaced 1-3 µm apart from each other (Błaszkowski, pers. observ.; Spain et al. 2006). Additionally, the upper surface of the spore wall layer 2 of the former fungus is slightly roughened and crazed, and that of the latter fungus is smooth. Moreover, in contrast to the spore wall layer 2 of Ap. appendicula staining orange (6A8) to high red (10A8) in Melzer's reagent, none of the spore wall layers of Ap. jimgerdemannii reacts in this reagent.
Another less important character separating the fungi compared here is the thickness of their inner germination walls. Both the germination walls 1 and 2 of spores of Ap. appendicula are much thicker (4.0-12.4 µm and 3.2-8.3 µm thick, respectively) than those of Ap. jimgerdemannii spores (2-3.5 µm and 4-6 µm thick, respectively; Błaszkowski, pers. observ.; Spain et al. 2006). Finally, glomoid spores have so far been recognized only in Ap. appendicula (Spain et al. 2006).
Compared with Ap. fennica and Ap. gerdemannii, the first germination wall of spores of only Ap. appendicula is ornamented (Błaszkowski 2003; Spain et al. 2006; Walker et al. 2007a). In the two latter species, the first germination wall is smooth.
According to Spain et al. (2006), Morton and Redecker (2001) mistakenly named Archaeospora leptoticha (N.C. Schenck & G.S. Sm.) J.B. Morton & D. Redecker and its glomoid morph using spores of Acaulospora appendicula Spain, Sieverd. & N.C. Schenck (now Ap. appendicula) and the known glomoid morph of this species. Acaulospora gerdemannii has not been known from pot cultures and its glomoid morph remains unknown. Consequently, Ar. leptoticha has been excluded from the genus Archaeospora, and Glomus leptotichum N.C. Schenck & G.S. Sm., erroneously considered a synanamorph of Ar. leptoticha and a synonym of Gl. fecundisporum N.C. Schenck & G.S. Sm., has been returned to the genus Glomus Tul. & C. Tul. (Spain et al. 2006). The same scientists also found Gl. fecundisporum to be not conspecific with Gl. leptotichum and, therefore, transferred the former species from Archaeospora to Glomus. In contrast, Walker et al. (2007b) considered Gl. fecundisporum and Gl. leptotichum to belong in the genus Appendicispora and erected two new combinations, Ap. fecundicispora (N.C. Schenck & G.S. Sm.) C. Walker, Vestberg & Schuessler and Ap. leptoticha (N.C. Schenck & G.S. Sm.) C. Walker, Vestberg & Schuessler.
REFERENCES
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http: //www.agro.ar.szczecin.pl/~jblaszkowski/.
Gai J. P., Christie P., Feng G., Li X. L. 2006. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16, 229-239.
Merryweather J., Fitter A. 1998. The arbuscular mycorrhizal fungi of Hyacinthoides non-scripta. I. Diversity of fungal taxa. New Phytol. 138, 117-129.
Morton J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus , based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Schalamuk S., Velazquez S., Chidichimo H., Cabello M. 2006. Fungal spore diversity of arbuscular mycorrhizal fungi associated with spring wheat: effect of tillage. Mycologia 98, 16-22.
Schenck N. C., Spain J. L., Howeler R. H. 1984. Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia . Mycologia 76, 685-699.
Spain J. L., Sieverding E., Oehl F. 2006. Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97, 163-182.
Walker C., Vestberg M., Demircik F., Stockinger H., Saito M., Sawaki H., Nishmura I., Schü฿ler A. 2007. Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol. Res. 111, 137-13.
Walker C., Vestberg M., Schü฿ler A. 2007. Nomenclatural clarifications in Glomeromycota. Mycol. Res. 111, 253-255.