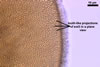
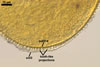
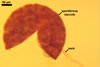
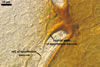
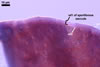
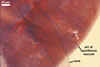
GERMINATION.
Not observed.
MYCORRHIZAE.
Many attempts to establish mycorrhizae of E. infrequens in one-species
cultures failed.
The only report of E. infrequens mycorrhizae established in a one-species culture probably is that of Sieverding and Toro (1986). According to these authors, E. infrequens formed typical vesicular-arbuscular mycorrhizae in roots of Pueraria phaseoloides Benth.
DISTRIBUTION.
Entrophospora infrequens has originally
been described as Gl. infrequens I.R. Hall from spores found in
New Zealand (Hall 1977). Ames and Schneider (1979) concluded that Gl. infrequens was
incompletely described and based on spores wet sieved from a celery field
soil of California
transferred this fungus to a newly established genus, Entrophospora
R.N. Ames & R.W. Schneid.
In Poland, E. infrequens
has been found in different both cultivated soils and those with natural
vegetation (Błaszkowski 1993a, b; Iwaniuk and Błaszkowski 2004). However,
this fungus occurred rarely and in low abundances.
As literature data and the observations of the author of this website indicate, E. infrequens has a worldwide distribution. It has been encountered in, e. g., Canada (Dalpé 1989), U.S.A. (Ames and Schneider 1979; Bloss and Walker 1987; Halvorson and Koske 1987; Hetrick and Bloom 1983; Koske and Halvorson 1989; Pfleger and Steward 1989; Schenck and Smith 1982; Stahl and Christensen 1982), Mexico (Sieverding and Oehl 2006), Colombia (Sieverding and Toro 1986), Bolivia (Sieverding and Oehl 2006), Brazil (Maia and Trufem 1990), Finland (Vestberg 1995), France, Switzerland and Germany (Oehl et al. 2003, 2004, 2005), Namibia (Uhlmann et al. 2004), Benin (Sieverding and Oehl 2006), Turkey (Błaszkowski, pers. observ.), India (Sridhar and Beena 2001), New Zealand (Hall 1977), and Australia (Hall and Abbott 1984).
NOTES.
Entrophospora infrequens, the type species of the genus Entrophospora, has originally erroneously been described as Glomus infrequens from incomplete spores with a short stalk resembling a subtending hypha of glomoid spores (Hall 1977). Ames and Schneider (1979) found identical spores, but formed inside the neck of a sporiferous saccule similar to the sporiferous saccules of Acaulospora spp. (Gerdemann and Trappe 1974). Consequently, they transferred this fungus to the newly erected genus Entrophospora in the family Endogonaceae Paoletti of the order Endogonales Moreau. Morton and Benny (1990) accommodated the genus Entrophospora in the family Acaulosporaceae. Walker and Schüßler (2004) included the Acaulosporaceae with the genera Acaulospora and Entrophospora in the new order Diversisporales. Recently, Sieverding and Oehl (2006) established the family Entrophosporaceae with the type genus Entrophospora, remaining in this genus only E. baltica and E. infrequens sensu Sieverding and Oehl (2006). Entrophospora colombiana and E. kentinensis, two other species classified within the genus Entrophospora sensu Ames and Schneider (1979), were transferred to the newly established genus Kuklospora and renamed K. colombiana and K. kentinensis.
The main reasons of the separation of Entrophospora spp. from the family Acaulosporaceae were (1) the formation of 2-walled spores by Entrophospora spp., and 3-walled ones by fungi of the Acaulosporaceae, (2) the presence of a relatively thick, coriaceous sensu Walker (1986), layer in the inner germinal wall of spores of Entrophospora spp. and its lack in any inner germinal wall of spores of the genera Acaulospora and Kuklospora, and (3) the production of an outer beaded layer in the innermost germinal wall of spores of most known species of the Acaulosporaceae compared with the lack of such a layer in the inner germinal wall of E. baltica and E. infrequens spores. Additionally, all available results of molecular analyses of members of the Acaulosporaceae and E. infrequens have indicated no relationship between these fungi (Sieverding and Oehl 2006).
Entrophospora infrequens and E. baltica are easy to distinguish. While the ornamentation of spores of the former fungus consists of tooth-shaped outgrowths, that of the former species forms small warts (Błaszkowski et al. 1998). Additionally, spores of E. baltica are surrounded with a hyphal mantle that is lacking in spores of E. infrequens.
REFERENCES
Ames R. N., Schneider R. W. 1979. Entrophospora, a new genus in the Endogonaceae. Mycotaxon 8, 347-352.
Błaszkowski J. 1989. Polish Endogonaceae. I. Acaulospora bireticulata, Entrophospora infrequens, Glomus caledonium, and Scutellispora pellucida. Karstenia 29, 1-10.
Błaszkowski J. 1993a. The occurrence of arbuscular fungi and mycorrhizae (Glomales) in plant communities of maritime dunes and shores of Poland. Bull. Pol. Ac. Sci. Biol. Sci. 41, 377-392.
Błaszkowski J. 1993b. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Błaszkowski J., Madej T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus fuegianum, two species in the Glomales from Poland. Mycotaxon 68, 165-184.
Bloss H. E., Walker C. 1987. Some endogonaceous mycorrhizal fungi of the Santa Catalina Mountains in Arizona. Mycologia 79, 649-654.
Dalpé Y. 1989. Inventaire et repartition de la flore endomycorhizienne de dunes et de rivages maritimes du Quebec, du Nouveau-Brunswick et de la Nouvelle-Ecosse. Naturaliste can. (Rev. Ecol. Syst.) 116, 219-236.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Brit. Mycol. Soc. 68, 341-356.
Hall I. R., Abbott L. K. 1984. Some Endogonaceae from south western Australia. Trans. Brit. Mycol. Soc. 83, 203-208.
Halvorson W. L., Koske R. E. 1987. Mycorrhizae associated with an invasion of Erechtites glomerata (Asteraceae) on San Miguel Island, California. Madrono 34, 260-268.
Hetrick B. A. D., Bloom J. 1983. Vesicular-arbuscular mycorrhizal fungi associated with native tall grass prairie and cultivated winter wheat. Can. J. Bot. 61, 2140-2146.
Koske R. E., Halvorson W. L. 1989. Mycorrhizal associations of selected plant species from San Miguel Island, Channel Islands National Park, California. Pacific Sci. 43, 32-40.
Iwaniuk A., Błaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part I. Occurrence of arbuscular fungi and mycorrhizae. Acta Mycol. 39(1), 59-84.
Maia L. C., Trufem S. F. B. 1990. Vesicular-arbuscular mycorrhizal fungi in cultivated soils in Pernambuco State, Brazil. Revista Brasil. Bot. 13, 89-95.
Morton J. B., Benny G. L. 1990. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 37, 471-491.
Oehl F., Sieverding E., Ineichen K., Mader P., Boller T., Wiemken A. 2003. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 69, 2816-2824.
Oehl F., Sieverding E., Mader P., Dubois D., Ineichen K., Boller T., Wiemken A. 2004. Impact of long-term conventional an organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 138, 574-583.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Pfleger F. L., Steward E. L. 1989. Survey of the Endogonaceae in Minnesota with synoptic keys to genera and species. J. Minnesota Ac. Sci. 54, 25-29.
Schenck N. C., Smith G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 74, 77-92.
Sieverding E., Oehl F. 2006. Revision of Entrophospora and description of Kuklospora and Intraspora, two new genera in the arbuscular mycorrhizal Glomeromycetes. J. Appl. Bot. Food Qual. 80, 69-81.
Sieverding E., S. Toro T. 1986. The genus Entrophospora in Colombia. In: Gianinazzi-Pearson V., Gianinazzi S. (eds). Physiological and genetical aspects of mycorrhizae. Proc. 1st European Symposium on Mycorrhizae. Dijon, 1-5 July 1985, 621-626.
Sridhar K. R., Beena K. R. 2001. Arbuscular mycorrhizal research in coastal sand dunes: a review. Proc. Nat. Acad. Sci. India. 71, 179-205.
Stahl P. D., Christensen M. 1982. Mycorrhizal fungi associated with Bouteloua and Agropyron in Wyoming sagebrush-grasslands. Mycologia 74, 877-885.
Uhlmann E., Görke C., Petersen A., Oberwinkler F. 2004. Arbuscular mycorrhizae from semiarid regions of Namibia. Can. J. Bot. 82, 645-653.
Walker C., Schüßler A. 2004. Nomenclatural clarifications and new taxa in the Glomeromycota. Mycol. Res. 108, 979-982.
Vestberg M. 1995. Occurrence of some Glomales in Finland. Mycorrhiza 5, 329-336.