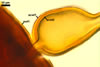
GERMINATION.
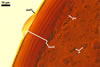
A germ tube develops from the germinal wall and penetrates the spore wall.
AUXILIARY
CELLS. No found.
MYCORRHIZAE.
Not established yet. According to Bentivenga and Morton (1995),
the mycorrhizae of Gi. margarita consisted of arbuscules and intraradical
hyphae staining darkly in trypan blue.
DISTRIBUTION.
In Poland, Gi. margrita was found in four mixtures of roots and rhizosphere soils, of which three were collected in the Lubuskie province and one in the Western Pomerania province. All the mixtures represented wild plants. These were Achillea millefolium L.,
Polygonum persicaria L.,
Rumex acetosella L., and Taxus baccata L. Of them, only
A. millefolium and
P. persicaria hosted spores of Gi. margarita in the field. However, the associations of Gi. margarita with roots of P. persicaria and
T. baccata were revealed only after the cultivation of their field-collected roots and rhizosphere soils in trap cultures. The sporulation of Gi. margarita in both the field and trap cultures was low. Among the 109 spores of arbuscular fungi isolated from 100 g of dry field soil taken under A. millefolium, only one (0.9%) belonged to Gi. margarita. Polygonum persicaria growing in the field hosted a total of 77 spores in 100 g dry soil, including two (2.6%) of Gi. margarita.
In trap cultures with Zea mays L. as the plant host and mixtures of roots and rhizosphere soils of
R. acetosella and T. baccata, the densities of Gi. margarita spores were 2 and 9 in 100 g dry soil, respectively, and Gi. margarita was the only arbuscular fungus revealed.
In the field, other species of arbuscular fungi associated with roots of A. millefolium were Glomus aggregatum, Gl. constrictum, and Scutellospora dipurpurescens, and Gl. constrictum and Gl. deserticola occurred among roots of P. persicaria.
Gigaspora margarita probably has a worldwide distribution. The holotype of this fungus has been selected from spores recovered from under Glycine max (L.) Merr. growing in a pot culture containing a root x rhizosphere soil mixture of Gly. max cultivated at the Agronomy South Farm of University of Illinois, USA (Becker and Hall 1976). Apart from many other reports of the occurrence of Gi. margarita in both cultivated (agricultural soils, orchards, nurseries) and natural sites (maritime sand dunes, native woodland, prairie) of the U.S. (An et al. 1993; Hetrick and Bloom 1983; Koske and Gemma 1997; Menge et al. 1977; Miller et al. 1985; Nicolson and Schenck 1979; Rose 1988; Schenck and Kinloch 1980; Schenck and Smith 1981), this species has also been found associated with different cultivated and uncultivated plants of Japan (Saito and Vargas 1991), New Zealand (Hall 1977), China (Wu et al. 2002), Canada (Marcel et al. 1988; Hamel et al. 1994), Mexico (Estrada-Torres et al. 1992), Cuba (Ferrer and Herrera 1980), and South America (Moreira-Souza et al. 2003; Sieverding 1989).
NOTES. Of the eight described species of the genus Gigaspora, Bentivenga and Morton (1995) accepted five. Gigaspora candida, Gi. ramisporophora, and Gi. tuberculata were considered congeneric with Gi. rosea, Gi. margarita, and Scutellospora persica, respectively.
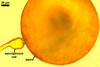
The most distinctive characters of Gi. margarita are its yellow-coloured spores, whose colour results from the pigmentation of the spore wall [is not associated with spore contents as in Gi. gigantea, and their relatively thin, usually less than 25 µm thick, wall.
The only other species of Gigaspora forming spores similar in size and colour to those of Gi. margarita is Gi. decipiens. The character separating the two fungi is the much thicker [(15-)20-45(-90) µm; Bentivena and Morton (1995)] spore wall of the former species.
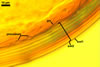
All attempts to establish one-species cultures of Gi. margarita made by the author of this website failed. Hence, the ontogenetic development and the differentiation of subcellular structures of spores of this fungus were not recognized. According to Bentivenga and Morton (1995), spores of Gi. margarita originate blastically from the top of a bulbous sporogenous cell and their wall consists of two, thin layers of near-equal thickness. At first, the spores are white. With time, the inner spore wall layer thickens and the spores darken because of the synthetization of new sublayers from the spore contents. The ontogenetic development of spores ends the formation of a germinal wall ornamented with processes prior to germination. The germ tube penetrates through the spore wall.
Bentivenga and Morton (1995) suggested that the two wall layers of spores of Gi. margarita and all the other Gigaspora spp. originating at the same time indicate them to be interdependent elements of one spore wall. A similar spore wall structure occurs in juvenile spores of members of the genus Scutellospora. This suggests that Gigaspora is ancestral to Scutellospora, the only other member of the family Gigasporaceae, although molecular analyses of ribosomal DNA has not confirmed it (Simon et al. 1993).
Compared with fungi of all the other genera of the phylum Glomeromycota, species of Gigaspora distinguish the lowest morphological variability of their spores. Spores of all Gigaspora spp. are smooth and the characters separating them are only colour and size of spores, as well as thickness of their wall. Except for Gi. gigantea, whose colour of spores results from the pigmentation of their contents, the spore colour of the other species comes from their wall. Bentivenga and Morton (1995) hypothesized that such low amount of diversity in Gigaspora results from two reasons. Firstly, germination of Gigaspora spp. is associated with spore wall and the energy remained after completion of this vital process probably is too low to aid further differentiation of this wall. In Scutellospora spp., germination is taken over by the germination shield, which enables the spore wall to unconstrainedly express variation. Secondly, the low level of variability in Gigaspora spp. may result from their recent separation and the lack of time to further diverge morphologically.
REFERENCES
An Z.-Q, Hendrix J. W., Hershamn D. E., Ferriss R. S., Henson G. T. 1993. The influence of crop rotation and soil fumigation on a mycorrhizal fungal community associated with soybean. Mycorrhiza 3, 171-182.
Becker W. N., Hall I. R. 1976. Gigaspora margarita, a new species in the Endogonaceae. Mycotaxon 4, 155-160.
Bentivenga S. P., Morton J. B. 1995. A monograph of the genus Gigaspora, incorporating developmental patterns of morphological characters. Mycologia 87, 719-731.
Estrada-Torres A., Varela L., Hernandez-Cuevas L., Gavito M. E. 1992. Algunos hongos micorrizicos arbusculares del estado de Tlaxcala, México. Rev. Mex. Mic. 8, 85-110.
Ferrer R. L., Herrera R. A. 1980. El genero Gigaspora Gerdemann et Trappe (Endogonaceae) en Cuba. Rev. Jar. Bot. Nac. Habana 1, 43-66.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol. Soc. 68, 341-356.
Hamel C., Dalpe Y., Lapierre C., Simard R. R., Smith D. L. 1994. Composition of the vesicular-arbuscular mycorrhizal fungi population in an old meadow as affected by pH, phosphorous and soil disturbance. Agric. Ecosyst. Environ. 49, 223-231.
Hetrick B. A. D., Bloom J. 1983. Vesicular-arbuscular mycorrhizal fungi associated with native tall grass prairie and cultivated winter wheat. Can. J. Bot. 61, 2140-2146.
Koske R. E., Gemma J. N. 1997. Mycorrhizae and succession in plantings of beachgrass in sand dunes. Am. J. Bot. 84, 118-130.
Marcel G. A., van der Heiden M. G. A., Klironomos J. N., Ursic M., Moutoglis P., Streitwolf-Engel R., Boller T., Wiemken A., Sanders I. R. 1988. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69-72.
Menge J. A., Nemec S., Davis R. M., Minassian V. 1977. Mycorrhizal fungi associated with citrus and their possible interactions with pathogens. Proc. Int. Soc. Citriculture 3, 872-876.
Miller D. D., Domoto P., Walker C. 1985. Mycorrhizal fungi at eighteen apple rootstock plantings in the United States. New Phytol. 100, 379-391.
Moreira-Souza M., Truem S. F. B., Gomes-da-Costa S. M. Cardoso E. J. B. N. 2003. Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza 13, 211-215.
Nicolson T. H., Schenck N. C. 1979. Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71, 178-198.
Rose S. 1988. Above and belowground community development in a maritime sand dune ecosystem. Plant and Soil 109, 215-226.
Saito M., Vargas R. 1991. Vesicular-arbuscular mycorrhizal fungi in some humus-rich Ando soils of Japan. Soil Microorg. 38, 3-15.
Schenck N. C., Kinloch R. A. 1980. Incidence of mycorrhizal fungi on six field crops in monoculture on a newly cleared woodland site. Mycologia 72, 445-456.
Schenck N. C., Smith G. S. 1981. Distribution and occurrence of vesicular-arbuscular mycorrhizal fungi on Florida agricultural crops. Soil and Crop Sci. Soc. Florida 40, 171-175.
Sieverding E. 1989. Ecology of VAM fungi in tropical ecosystems. Agric., Ecosyst. and Environ. 29, 369-390.
Simon L., Bousquet T., Lévesque R. C., Lalonde M. 1993. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363, 67-69.
Wu T., Hao W., Lin X., Shi Y. 2002. Screening of arbuscular mycorrhizal fungi for the revegetation of eroded red soils in subtropical China. Plant and Soil 239, 225-235.