 |
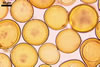 |
SPORES yellowish white (4A2) when young, deep orange (5A7-8) at maturity, to golden yellow (5B8) when older; globose to subglobose; (70-)98(-120) µm diam; sometimes ovoid; 80-120 x 110-150 µm; with a single subtending hypha.
Błaszk. V. Blanke, C. Renker & F. Buscot
 |
 |
SPORES yellowish white (4A2) when young, deep orange (5A7-8) at maturity, to golden yellow (5B8) when older; globose to subglobose; (70-)98(-120) µm diam; sometimes ovoid; 80-120 x 110-150 µm; with a single subtending hypha.
SUBCELLULAR STRUCTURE OF SPORES consists of one wall including three layers (swl1-3).
Layer 1 permanent, flexible to semiflexible, smooth, hyaline, (0.7-)1.0(-1.5) µm thick, sometimes ballooning, and then extending up to 30 µm from layer 1 in spores mounted in PVLG. This layer frequently accumulates a granular material, up to 1.5-7.0 µm thick, composed of soil debris.
Layer 2 laminate, smooth, yellowish white (4A2) to golden yellow (5B8), (2.7-)5.8(-8.8) µm thick.
Layer 3 flexible, smooth, hyaline, (0.5-)0.7(-1.3) µm thick, usually tightly adherent to layer 2 and almost always inseparably attached to the inner surface of subtending hyphal wall layer 2, close at the spore base to form a curved septum in the lumen of the subtending hypha.
None of the spore wall layers reacts in Melzer’s reagent.
In PVLG+Melzer's reagent |
In PVLG |
||||
Wall of subtending hypha yellowish white (4A2) to golden yellow (5B8); (0.7-)2.0(-4.7) µm thick at the spore base; composed of three layers continuous with spore wall layers 1-3; layers 1 and 3 extend up to 10.0 and 2.0-3.4 µm, respectively, below the spore base.
Pore occluded by a septum, (2.0-)2.9(-4.2) µm wide, continuous with spore wall layer 3 and occasionally also by a septum formed by a few innermost laminae of spore wall layer 2, positioned 3.0-6.5 µm below the spore base.
GERMINATION. Not observed.
MYCORRHIZAE. In one-species cultures with Zea mays L. as the plant host, mycorrhizae of Gl. aurantium consisted of arbuscules, vesicles, as well as intra- and extraradical hyphae (Błaszkowski et al. 2004). Arbuscules generally were numerous and evenly distributed along root fragments. Vesicles occurred very abundantly and were globose to subglobose; (18-)32(-45) µm diam; sometimes ellipsoid; 20-40 x 55-110 µm. Intraradical hyphae were (1.0-)4.8(-7.4) µm wide and grew parallel to the root axis. They were straight or slightly curved, sometimes formed Y- or H-shaped branches and coils. The coils were 12.5-50.0 x 22.5-140.0 µm. Extraradical hyphae were (2.5-)4.0(-4.7) µm wide. Their abundance varied, depending on the root fragments examined. In 0.1% trypan blue, arbuscules stained violet white (16A2) to lilac (16B4), vesicles violet white (19A2) to deep blue (19E6), intraradical hyphae violet white (16A2) to reddish violet (16C8), coils pale violet (16A3) to reddish violet (16B6), and extraradical hyphae light lilac (16A5) to royal purple (16D8).
In roots of Z. mays |
||||||||
Fig. 1 |
Fig. 2 |
PHYLOGENETIC POSITION. The analysis of the 5.8S gene within the ITS region revealed a close relationship between Gl. aurantium and Gl. versiforme (P. Karsten) S.M. Berch (Fig. 1). In the LSU tree, G. aurantium is the terminal taxon of a not supported lineage placed sister to the Glomus Group A (Fig. 2). Comparing the full length ITS sequences of Gl. aurantium with sequences assigned to G. versiforme available in GenBank, the molecular similarity of the two fungi ranged from 89 to 92%.
DISTRIBUTION. Glomus aurantium was discovered in a trap culture with a rhizosphere soil of Cenothera drummondi Hook colonizing dunes of the Mediterranean Sea adjacent to Tel-Aviv (32º4’N, 34º46’E) in December 1997 (Błaszkowski et al. 2004). This fungus also sporulated in 60 other trap cultures with dune rhizosphere soils of Ammophila arenaria (L.) Link and C. drummondi sampled near Tel-Aviv in 1997 (9 samples) and 2000 (51). Later, spores of Gl. aurantium were isolated from eight trap cultures established from soils collected under A. arenaria growing near Cape Salinas (36º19’N, 3º2’E) and Sóller (39º46’N, 2º4º’E), Majorca, Spain, and seven trap cultures containing rhizosphere soil and root mixtures taken from under A. arenaria growing in dunes adjacent to Calambrone (43º35’N, 10º18’E), Italy.
The arbuscular fungi accompanying Gl. aurantium in the field were Gl. constrictum Trappe and Gl. coronatum Giovann. The fungi co-occurring with Gl. aurantium in trap cultures with Israeli soils were Acaulospora paulinae Blaszk., Archaeospora trappei (R.N. Ames & Linderman) J.B. Morton & D. Redecker, Gl. arenarium Blaszk. et al., Gl. constrictum, Gl. coronatum, Gl. corymbiforme Blaszk., Gl. gibbosum Blaszk., Gl. mosseae (Nicol. & Gerd.) Gerd. & Trappe, Gl. xanthium Blaszk. et al., an undescribed Glomus sp., Pacispora scintillans (S.L. Rose & Trappe) Sieverd. & Oehl, S. calospora (Nicol. & Gerd.) C. Walker & F.E. Sanders, and S. persica (Koske & C. Walker) C. Walker & F.E. Sanders. Apart from Gl. aurantium, the Majorca’s cultures contained Gl. constrictum, Gl. coronatum, Gl. macrocarpum Tul. & C. Tul., two undescribed Glomus spp., Pac. scintillans (S.L. Rose & Trappe) Sieverd. & Oehl, S. calospora, and S. persica. The cultures representing Italy still hosted Ac. scrobiculata Trappe, Entrophospora schenckii Sieverd. & S. Toro, Gl. constrictum, two undescribed Glomus spp., Pac. scintillans (S.L. Rose & Trappe) Sieverd. & Oehl, S. fulgida Koske & C. Walker, and S. persica.
NOTES. Glomus aurantium most distinguishes its orange-coloured spores and their wall structure. In the 3-layered spore wall, the structures most distinctive are the flexible to semi-flexible permanent outermost layer 1, which sometimes balloons in lactic acid-based mountants, and the flexible innermost layer 3. None of the three layers stains in Melzer’s reagent.
When viewed under a dissecting microscope, spores of Gl. aurantium most resemble those of Gl. pustulatum Koske et al. and Gl. versiforme because of their orange pigmentation and a similar size range (Koske et al. 1986; Morton 2002). Younger, yellow-coloured spores of Gl. aurantium are also reminiscent of those of Gl. claroideum N.C. Schenck & G.S. Sm., Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. lamellosum Dalpé et al., and Gl. luteum L.J. Kenn. et al.
Examination of spores crushed in PVLG and PVLG mixed with Melzer’s reagent under a compound microscope readily separates Gl. aurantium from the six species listed above. Although both Gl. aurantium and Gl. pustulatum produce spores with a wall consisting of three permanent layers, the outermost layer of the former species is smooth or covered with granular debris and that of Gl. pustulatum is ornamented with blistery, cup- or irregularly-shaped processes (Koske et al. 1986).
The character most distinguishing Gl. versiforme from Gl. aurantium is the lack of the innermost flexible spore wall layer of the latter species (Morton 2002). Additionally, the outermost spore wall layer in Gl. aurantium is permanent, and that of Gl. versiforme degrades with age.
The spore wall of Gl. fasciculatum and Gl. lamellosum also consists of three layers (Błaszkowski et al. 2002; Dalpé et al. 1992; Walker and Koske 1987). Moreover, in Gl. fasciculatum, these layers are persistent and have similar phenotypic properties as those of Gl. aurantium. However, two outer spore wall layers of the former species stain red in Melzer’s reagent, whereas all three wall layers of Gl. aurantium spores remain non-reactive in this reagent. Additionally, the outermost spore wall layer of Gl. fasciculatum does not accumulate debris as that of Gl. aurantium and spores of the former fungus frequently occur in aggregates (vs. only single spores in Gl. aurantium).
In contrast to the outermost persistent layer and the innermost in-amyloid layer of spore wall of Gl. aurantium, the outermost wall layer of spores of Gl. lamellosum degrades with age and their innermost layer stains pinkish in Melzer’s reagent (Błaszkowski et al. 2002; Dalpé et al. 1992; Morton 2002).
Glomus aurantium differs from Gl. claroideum and Gl. luteum in the number of spore wall layers, as well as in their phenotypic and biochemical properties. Spores of Gl. claroideum and Gl. luteum have a penultimate laminate wall layer and an innermost flexible wall layer of spores of Gl. aurantium (Błaszkowski et al. 2003; Kennedy et al. 1999; Schenck and Smith 1982; Stürmer and Morton 1997; Walker and Vestberg 1998). However, the laminate layer in Gl. aurantium is surrounded with only one permanent layer, whereas that of the former two species is covered with two layers, of which each degrades and sloughs with age. Finally, the outermost spore wall layer of Gl. claroideum and Gl. luteum stains pink to purplish red in Melzer’s reagent, but remains non-reactive in Gl. aurantium.
The accumulation of soil debris on the surface of mature spores of Gl. aurantium is a property that also distinguishes spores of Gl. viscosum Nicol., another species producing spores of a similar structure of their wall and a size range (Morton 2002; Walker et al. 1995). However, spores of Gl. viscosum are much lighter-coloured (subhyaline to pale yellow vs. yellowish white to golden yellow in Gl. aurantium) and frequently occur in aggregates (vs. only singly in G. aurantium).
The molecular analysis of the 5.8S gene confirmed a close relationship of Gl. aurantium with Gl. versiforme. Based on sequence data from the ribosomal small subunit (SSU), Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler and Gl. etunicatum W.N. Becker & Gerd. (isolate W3239/Att382-16) also clustered phylogenetically next to Gl. versiforme (Schüßler et al. 2001). However, Schüßler et al. (2001) showed some SSU sequences of Gl. etunicatum (isolate UT 316) that rather clustered in Glomus Group B, which was also the case for ITS sequences of Gl. etunicatum from GenBank used in our study. Unfortunately, LSU sequence data of D. spurca, Gl. versiforme, and Gl. etunicatum to further assess and confirm the phylogenetic position of Gl. aurantium are missing and therefore Gl. aurantium formed a single lineage in the LSU phylogenetic tree presented here. Finally, comparing morphological properties of the four fungi listed above, only Gl. aurantium produces spores having an innermost flexible layer in their wall structure (Morton 2002).
Glomus aurantium is probably adapted to warm soils of southern hemisphere. They have not been found in any of ca. 3000 soil samples collected in different dune and non-dune soils of northern Europe (Błaszkowski 2003). Koske (1987) found temperature to be the main abiotic factor influencing the structure of arbuscular fungi of the barrier dunes extending from New Jersey to Virginia. According to Pirozynski (1968), temperature is the major factor determining the distribution and occurrence of fungi in general.
REFERENCES
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Błaszkowski J., Blanke V., Renker C., Buscot F. 2004. Glomus aurantium and G. xanthium, new species in Glomeromycota. 90, 447-467.
Błaszkowski J., Adamska I., Madej T. 2002. Glomus lamellosum (Glomales, Zygomycota), an arbuscular mycorrhizal fungal species new for Poland and Europe. Mycotaxon 81, 281-292.
Błaszkowski J., Adamska I., Czerniawska B. 2003. Glomus claroideum and G. spurcum, arbuscular mycorrhizal fungi (Glomeromycota) new for Poland and Europe, respectively. Acta Soc. Bot. Pol. 72, 149-156.
Dalpé Y., Koske R. E., Tews L. L. 1992. Glomus lamellosum sp. nov.: a new Glomaceae associated with beach grass. Mycotaxon 43, 289-293.
Kennedy L. J., Stutz J. C., Morton J. B. 1999. Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91, 1083-1093.
Koske R. E. 1987. Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia 79, 55-68.
Koske R. E., Friese C., Walker C., Dalpé Y. 1986. Glomus pustulatum: A new species in the Endogonaceae. Mycotaxon 26, 143-149.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University. http://www.invam.caf.wvu.edu/.
Pirozynski K. A. 1968. Geographical distribution of fungi. In: G. C. Ainsworth, A. S. Sussman. The fungi. Academic Press. New York, pp. 487-504.
Schenck N. C., Smith G. S. 1982. Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 74, 77-92.
Schüßler A., Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105, 1413-1421.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Walker C., Giovannetti M., Avio L., Citernesi A. S., Nicolson T. H. 1995. A new fungal species forming arbuscular mycorrhizas: Glomus viscosum. Mycol. Res. 99, 1500-1506.
Walker C., Koske R. E. 1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum redescribed. Mycotaxon 30, 253-262.
Walker C., Vestberg M. 1998. Synonymy amongst the arbuscular mycorrhizal fungi: Glomus claroideum, G. maculosum, G. multisubstensum and G. fistulosum. Ann. Bot. 82, 601-624.