
(Nicol. & Gerd.) Trappe & Gerd.
 |
SPORES single in the soil; pale yellow (3A3) to golden yellow (5B8); globose to subglobose; (90-)224(-370) µm diam; with a single subtending hypha.
SUBCELLULAR STRUCTURE OF SPORES consists of one wall with four layers (swl 1-4).
 |
 |
 |
 |
 |
 |
In PVLG |
|||||
Layer 1 mucilaginous, hyaline, (0.8-)1.0(-2.5) µm thick, sometimes staining reddish white (7A2) in Melzer’s reagent.
Layer 2 rigid, hyaline, (1.0-)1.8(-3.5) µm thick.
Layer 3 fragile, disintegrating into small fragments in crushed spores, hyaline, (0.6-)1.5(-3.8) µm thick.
Layer 4 laminate, pale yellow (3A3) to golden yellow, (5B8), (3.8-)5.5(-7.5) µm thick.
Most juvenile spores have a wall with layers 1 and 2 only. Layers 3 and 4 develop successively during maturation of spores.
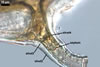
SUBTENDING HYPHA pale yellow (3A3) to golden yellow (5B8); straight or curved; cylindrical, sometimes funnel-shaped or constricted at the spore base; (14.5-)19.0(-26.0) µm wide.
 |
 |
In PVLG |
|
Pore closed by a curved septum continuous with the innermost laminae of the laminate spore wall layer 4.
GERMINATION. A germ tube emerges from the lumen of the subtending hypha.
 |
 |
 |
 |
 |
In roots of P. lanceolata |
||||
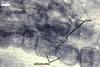
MYCORRHIZAE. In roots of Plantago lanceolata L., mycorrhizae of Gl. caledonium consisted of arbuscules, vesicles, as well intra- and extraradical hyphae staining intensively in 0.1% trypan blue.
DISTRIBUTION. Glomus caledonium is one of the most frequently occurring arbuscular fungi in Poland (Błaszkowski 1989, 1993; Iwaniuk and Błaszkowski 2004). It abundantly sporulated both in poor and rich soils.
Literature data evidence that Gl. caledonium has a worldwide distribution. It has been found in, e. g., many states of the U. S. A. (Gerdemann and Trappe 1974; Koske 1987, Miller et al. 1986; Pfleger and Steward 1989), Scotland (Nicolson and Gerdemann 1968), Israel (Błaszkowski et al. 2001), India (Selvaraj and Subramanian 1987), Taiwan (Wu and Chen 1986), New Zealand (Hall 1977), and Australia (Hall and Abbott 1984; McGee 2002).
NOTES. Morton (1996) redescribed Gl. caledonium and the defined spore characters completely agree with those presented here. When observed under a dissecting microscope, spores of Gl. caledonium most resemble those of Gl. mosseae (Nicol. & Gerd.) Gerd. & Trappe. Examination of the subcellular structure of spores of these species readily separates them. Glomus caledonium produces spores with four layers, and Gl. mosseae with three layers of different phenotypic properties.
REFERENCES
Błaszkowski J. 1989. Polish Endogonaceae 1. Acaulospora bireticulata, Entrophospora infrequens, Glomus caledonium, and Scutellospora pellucida. Karstenia 29, 1-10.
Błaszkowski J. 1993. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Błaszkowski J., Tadych M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz. Inz. Ksztalt. Srod. 22, 8-27.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Iwaniuk A., Błaszkowski J. 2004. Arbuscular fungi and mycorrhizae of agricultural soils of the Western Pomerania. Part I. Occurrence of arbuscular fungi and mycorrhizae. Acta Mycol. 39(1), 59-84.
Koske R. E. 1987. Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia 79, 55-68.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol. Soc. 68, 341-356.
Hall I. R., Abbott L. K. 1984. Some Endogonaceae from south western Australia. Trans. Br. Mycol. Soc. 83, 203-208.
McGee P. A., Trappe J. M. 2002. The Australian zygomycetous mycorrhizal fungi. II. Further Australian sporocarpic Glomaceae. Aust. Sys. Bot. 15, 115-124.
Miller D. D., Domoto P. A., Walker C. 1986. Mycorrhizal fungi at eighteen apple rootstock plantings in the United States. New Phytol. 100, 379-391.
Morton J. M. 1996. Redescription of Glomus caledonium based on correspondence of spore morphological characters in type specimens and a living reference culture. Mycorrhiza 6, 161-166.
Morton J. B. 2000. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. West Virginia University.
Nicolson T. H., Gerdemann J. W. 1968. Mycorrhizal Endogone species. Mycologia 60, 313-325.
Pfleger F. L., Steward E. L., 1989. Survey of the Endogonaceae in Minnesota with synoptic keys to genera and species. J. Min- nesota Ac. Sci. 54, 25-29.
Selvaraj T., Subramanian G. 1987. Vesicular-arbuscular mycorrhizal fungi in roots and scale-like leaves of Acorus calamus Linn. and Calacasia esculenta Linn. Cur. Sci. 56, 1112- 1114.
Wu C.-G., Chen Z. C. 1986. The Endogonaceae of Taiwan. I. A preliminary investigation on Endogonaceae of babmbo vegetation at Chi-Tou areas, Central Taiwan. Taiwania 31, 65-88.