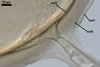
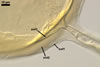
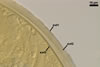
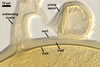
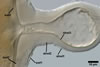
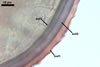
GERMINATION.
A
germ tube emerges from the lumen of the subtending hypha.
MYCORRHIZAE.
In one-species cultures with the host plant Plantago lanceolata
L., mycorrhizae of Gl. clarum consisted of arbuscules, vesicles,
as well as intra- and extraradical hyphae staining intensively in 0.1% trypan
blue.
DISTRIBUTION.
In Poland,
Gl. clarum has occurred in maritime dunes of Swinoujscie (53º55’N,
14º14’E; Błaszkowski 1994), the Vistula Bar (54o21’N, 19o14’E;
Błaszkowski et al. 2002a), and in inland dunes of the Bledowska Desert (50o22’N,
19o34’E; Błaszkowski et al. 2002b).
Glomus clarum has also been found in cultivated and uncultivated
soils of Florida, Kansas, Kentucky, West Virginia, U.S.A. (Day et al. 1987;
Hetrick and Bloom 1983; Miller et al. 1985; Morton 1985; Nicolson and Schenck
1979; Schenck and Kinloch 1980; Schenck and Smith 1981), Colombia, South America
(Schenck et al. 1984; Sieverding 1989), Adelaide, South Australia (McGee 1986),
Singapore (Louis and Lim 1988) and in soils of maritime dunes and shores of
Quebec, New Brunswick, New Scotia, Canada (Dalpé 1989), and Madras,
India (Mohankumar et al. 1988).
NOTES.
Glomus clarum most resembles Gl.
lamellosum Dalpé et al. and Gl. luteum L.J. Kenn. et
al. due to the hallo formed by the relatively thick and colourless layer adherent
to the coloured structural layer of its spores (Błaszkowski et al. 2002; Dalpé
et al. 1992; Kennedy et al. 1999). However, in Gl. lamellosum, this
thick and colourless layer is uniform and sloughs with age, and is not a laminate
and permanent as in Gl. clarum. Additionally, Gl. lamellosum
and Gl. luteum still have a fourth, very thin and flexible layer
that is absent in spores Gl. clarum.
REFERENCES
Błaszkowski J. 1994.
Glomus clarum (Glomales, Zygomycetes), a new vesicular-arbuscular
fungus to Poland. Mycotaxon 52, 99-107.
Błaszkowski J., Adamska
I., Madej T. 2002. Glomus lamellosum (Glomales, Zygomycota), an arbuscular
mycorrhizal fungal species new for Poland and Europe. Mycotaxon 81, 281-292.
Błaszkowski J., Adamska
I., Czerniawska B. 2002a. Arbuscular mycorrhizal fungi (Glomeromycota) of
the Vistula Bar. Acta Mycol. 37, 39-62.
Błaszkowski J., Tadych
M., Madej T. 2002b. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of
the Bledowska Desert, Poland. Acta Soc. Bot. Pol. 71, 71-85.
Dalpé Y. 1989.
Inventaire et repartition de la flore endomycorhizienne de dunes et de rivages
maritimes du Quebec, du Nouveau-Brunswick et de la Nouvelle-Ecosse. Naturaliste
can. (Rev. Ecol. Syst.) 116, 219-236.
Dalpé Y., Koske
R. E., Tews L. L. 1992. Glomus lamellosum sp. nov.: a new Glomaceae
associated with beach grass. Mycotaxon 43, 289-293.
Day L. D., Sylvia D.
M., Collins M. E. 1987. Interactions among vesicular-arbuscular mycorrhizae,
soil, and landscape position. Soil Sci. Am. J. 51, 635-639.
Hetrick B. A. D., Bloom
J. 1983. Vesicular-arbuscular mycorrhizal fungi associated with native tall
grass prairie and cultivated winter wheat. Canad. J. Bot. 61, 2140-2146.
Kennedy L. J., Stutz
J. C., Morton J. B. 1999. Glomus eburneum and G. luteum,
two new species of arbuscular mycorrhizal fungi, with emendation of G.
spurcum. Mycologia 91, 1083-1093.
Louis I., Lim G. 1988.
Observations on in vitro sporulation of Glomus clarum. Trans. Brit.
Mycol. Soc. 91, 698-699.
McGee P. A. 1986. Further
sporocarpic species of Glomus (Endogonaceae) from South Australia.
Trans. Brit. Mycol. Soc. 87, 123-129.
Miller D. D., Domoto
D. P., Walker C. 1985. Mycorrhizal fungi at eighteen apple rootstock plantings
in the United States. New Phytol. 100, 379-391.
Mohankumar V., Ragupathy
S., Niemala C. B., Mahadevan A. 1988. Distribution of vesicular-arbuscular
mycorrhizae (VAM) in the sandy beach soils of Madras coast. Current Sci. 57,
367-368.
Morton J. B. 1985. Variation
in mycorrhizal and spore morphology of Glomus occultum and Glomus
diaphanum as influenced by plant host and soil environment. Mycologia
77, 192-204.
Nicolson T. H., Schenck
N. C. 1979. Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71,
178-198.
Schenck N. C., Kinloch
R. A. 1980. Incidence of mycorrhizal fungi on six field crops in monoculture
on a newly cleared woodland site. Mycologia 72, 445-456.
Schenck N. C., Smith
G. S. 1981. Distribution and occurrence of vesicular-arbuscular mycorrhizal
fungi on Florida agricultural crops. Soil and Crop Sci. Soc. Florida, Proc.
40, 171-175.
Schenck N. C., Spain
J. L, Sieverding E., Howeler R. H. 1984. Several new and unreported vesicular-arbuscular
mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76, 685-699.
Sieverding E. 1989. Ecology
of VAM fungi in tropical agrosystems. Agric., Ecosyst. and Environment. 29,
369-390.