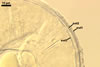
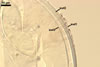
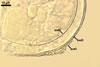
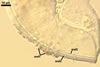
GERMINATION.
Not observed.
MYCORRHIZAE.
According to Morton and Walker (1984) and Morton (2000), mycorrhizae of Gl. diaphanum comprise arbuscules, vesicles, and hyphae. Late mycorrhizal colonization mainly consists of intraradical hyphae and aggregates of intraradical spores. All the structures stain darkly in trypan blue.
DISTRIBUTION.
The spores of Gl.
diaphanum presented here come from Dr. J. B. Morton, West Virginia University, USA.
The author of this website has never found spores of Gl. diaphanum in soils
of Poland, as well as in Asia, Africa, and other regions of Europe.
Glomus diaphanum probably has a worldwide distribution, although an exceptionally few data inform of its finding.
The holotype of Gl. diaphanum comes from a trap culture started with a root-soil mixture sampled from the rhizosphere of red clover (Trifolium pratense L.) growing on a partially reclaimed coal surface maine located in Elkins, Randolf County, West Virginia, U.S.A. Additionally, this fungus has been found in many other cultivated and uncultivated soils of the Western Virginia (Morton 1985). Most of these soils were moderately to highly acidic.
The only reports of the occurrence of Gl. diaphanum in Europe are those of Oehl et al. (2003, 2005), which inform of the presence of this fungus at many cultivated sites of the Upper Rhine Valley between Basel (Switzerland), Freiburg i. Br. (Deutschland), and Mulhouse (France).
Additionally, Zhang and Wang (1991) found Gl. diaphanum spores in Beijing and Xinjiang areas located in China.
NOTES.
The arbuscular fungi producing spores most resembling those of Gl. diaphanum are Gl. viscosum Nicol., Paraglomus laccatum (Blaszk.) C. Renker, Blaszk. & F. Buscot, and Par. occultum (C.
Walker) J.B. Morton & D. Redecker. Spores of all these species are glomoid, hyaline and of a similar size
range (Błaszkowski 1988; Morton 2002; Morton and Redecker 2001; Morton
and Walker 1984;
Renker et al, in press; Walker et al.
1995). However, none of these fungi forms the flexible to semiflexible
innermost wall layer of Gl. diaphanum spores and their outermost mucilagenous
layer staining in Melzer's reagent. Additionally, most spores of Gl.
diaphanum occur singly in the soil, whereas those of Gl. viscosum are
usually formed in loose aggregates branching from a common hypha (Morton
2002; Walker et al. 1995).
REFERENCES
Błaszkowski J. 1988.
Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland.
Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Morton J. B. 1985. Variation in mycorrhizal and spore morphology of Glomus occultum and Glomus diaphanum as influenced by plant host and soil environment. Mycologia 77, 192-204.
Morton J. B. 2002. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Morton
J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and
Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on
concordant molecular and morphological characters. Mycologia 93, 181-195.
Morton J. B., Walker
C. 1984. Glomus diaphanum: a new species in the Endogonaceae common
to West Virginia. Mycotaxon 21, 431-440.
Oehl F., Sieverding E., Ineichen K., Mader P., Boller T., Wiemken A. 2003. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 69, 2816-2824.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Renker C., Błaszkowski J., Buscot F. Paraglomus laccatum comb. nov. - a new member of Paraglomeraceae (Glomeromycota). Nova Hedwigia (in press).
Walker C., Giovannetti
M. Avio L., Citernesi A. S., Nicolson T. H. 1995. A new fungal species forming
arbuscular mycorrhizas: Glomus viscosum. Mycol. Res. 99, 1500-1506.
Zhang M-Q., Wang Y-S. 1991. Seven species of VA mycorrhizal fungi from northern China. Acta Mycol. Sin. 10, 13-21.