GERMINATION.
Not observed.
MYCORRHIZAE.
In the field, Gl. laccatum has been associated with vesicular-arbuscular
mycorrhizal roots of Ammophila arenaria (L.) Link, Helictotrichon
pubescens (Huds.) Pilg., and an undetermined Festuca sp. (Błaszkowski
1988; Błaszkowski et al. 2002; Tadych and Błaszkowski 2000b; Iwaniuk and Błaszkowski,
unpubl.). Many attempts to establish Gl. laccatum mycorrhizae in
one-species cultures with Plantago lanceolata L. as the plant host
failed.
DISTRIBUTION.
Glomus laccatum has
infrequently been recorded in Poland. Błaszkowski (1988) described this fungus
from spores revealed in the rhizosphere soil of Festuca sp. growing
in Jastrzebia Góra (55o18’N, 17o54’E). Later, Błaszkowski
(1994) and Tadych and Błaszkowski (2000a) found its presence among roots
of A. arenaria and H. pubescens growing in the Hel Peninsula
(54o36'-54o47'N, 18o25'-18o48'E) and the Slowinski National Park (54o45'N,
17o26'E). However, other investigations with trapping arbuscular fungi in
pot cultures indicated that Gl. laccatum is a relatively frequent
inhabitant of both cultivated and uncultivated sites of Poland (Błaszkowski
et al. 2002; Tadych and Błaszkowski 2000b; Iwaniuk and Błaszkowski, unpubl.).
Walker (pers. comm.)
found this fungus in soils of the Great Britain. The infrequent disclosures
of Gl. laccatum in field-collected soil samples may result from the
lack or irregular sporulation of this fungus in the field conditions or a
low persistency of its spores. In the field, a great part of arbuscular fungi
either do not sporulate at all or their sporulation is infrequent and seasonal
(Stürmer and Bellei 1994; Stutz and Morton 1996). Glomus laccatum
forms small, hyaline spores with a delicate spore wall that may easily be
decomposed by soil microorganisms. Many soil microorganisms are parasites
of AMF (Lee and Koske 1994).
NOTES.
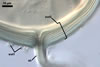
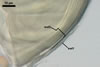
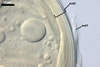
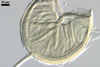
Apart from Gl. laccatum, other species of arbuscular fungi forming glomoid hyaline spores are Gl.
diaphanum J.B. Morton & C. Walker, Gl. viscosum Nicol.,
and Paraglomus occultum (C. Walker) J.B. Morton & D. Redecker.
The spore wall of Gl.
diaphanum consists of three layers with a flexible innermost layer (Morton
and Walker 1984; Morton 2000), whose lacks Gl. laccatum. Spores
of Gl. viscosum occur in loose aggregates (Walker et al. 1995), and
not singly in the soil as those of Gl. laccatum. Additionally, the
spore wall of Gl. viscosum consists of three layers (Morton 2000),
whereas that of Gl. laccatum contains two layers. Paraglomus
occultum produces spores with three uniform layers (vs. two, phenotypically
different layers in Gl. laccatum).
REFERENCES
Błaszkowski J. 1988.
Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland.
Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Błaszkowski J. 1994.
Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland.
Mycorrhiza 5, 71-88.
Błaszkowski J., Adamska
I., Czerniawska B. 2002. Arbuscular mycorrhizal fungi (Glomeromycota) of the
Vistula Bar. Acta Mycol. 37, 39-62.
Morton J. B. 2000. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Lee P. J., Koske R.
E. 1994. Gigaspora gigantea: parasitism of spores by fungi and actinomycetes.
Mycol. Res. 98, 458-466.
Morton J. B., Walker
C. 1984. Glomus diaphanum: a new species in the Endogonaceae common
to West Virginia. Mycotaxon 21, 431-440.
Stutz J. C., Morton
J. B. 1996. Successive pot cultures reveal high species richness of arbuscular
mycorrhizal fungi in arid ecosystems. Can. J. Bot. 74, 1883-1889.
Stürmer S. L., Bellei
M. M. 1994. Composition and seasonal variation of spore populations of arbuscular
mycorrhizal fungi in dune soils on the island of Santa Catarina, Brazil. Can.
J. Bot. 72, 359-363.
Tadych M., Błaszkowski
J. 2000a. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.
Tadych M., Błaszkowski
J. 2000b. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola
Forests. Acta Mycol. 35, 3-23.
Walker C., Giovannetti
M., Avio L., Citernesi A. S., Nicolson T. H. 1995. A new fungal species forming
arbuscular mycorrhizas: Glomus viscosum. Mycol. Res. 99, 1500-1506.