Glomus
nanolumen
Koske & Gemma
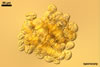
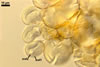
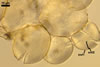
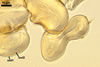
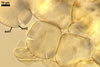
Spores formed in sporocarps and singly in the soil.
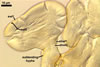
Sporocarps
subglobose; 90-250 µm diam; to irregular; composed of ca. 5-40 loosely to tightly packed spores.
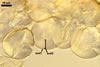
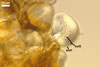
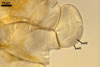
SPORES produced blastically at the tip of hyphae continuous with hyphae of the inside of sporocarps or mycorrhizal extraradical hyphae (when single).
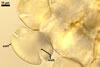
Spores hyaline, yellowish white (1A2) to pale yellow (4A3); subglobose; (13-)36(-43) µm diam; pyriform, ovoid to irregular; 20-53 x 25-73 µm; with one subtending hypha.
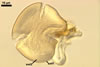
SUBCELLULAR
STRUCTURE OF SPORES
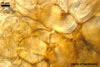
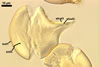
consists of a spore wall comprising two layers
(swl1 and 2).
Layer
1,
forming the spore surface, permanent, unit, hyaline, yellowish white (1A2) to pale yellow (4A3), (0.7-)0.9(-1.2) µm thick.
GERMINATION.
Unknown.
MYCORRHIZAE. Spores of Gl. nanolumen have been associated with roots of Scaevoia sericea Vahl. and Ipomoea stolonifera (Cyrill.) J.F. Gmel. (Koske and Gemma 1989). However, the characters of mycorrhizae from one-species cultures of this fungus remain unknown to date.
DISTRIBUTION. The type of Gl. nanolumen has been designed from spores isolated from under S. sericea colonizing calcareous sand dunes of the Polihale State Park located on Kauai, Hawaii (Koske and Gemma 1989). The dunes on Kauai are the only so far known sites of the occurrence of this fungus (Koske and Gemma 1989, 1996).
NOTES.
The unique structure of Gl. nanolumen is its very uneven in thickness laminate spore wall layer 2. In some regions, the inner surface of this layer almost riches the spore centre, remaining a small space filled with cytoplasm. The space usually is less than 50% of the spore diameter and in some specimens it does not exceed 20% (Koske and Gemma 1989).
Another distinctive property of this layer is its fragility. In vigorously crushed spores, this layer frequently cracks, appearing many radial fracture lines. However, this layer does not break into separate pieces. In contrast, in most other Glomus spp., a laminate layer of vigorously crushed spores usually releases single laminae or their groups (Błaszkowski, pers. observ.).
When observed under a dissecting microscope, sporocarps of Gl. nanolumen are reminiscent of those of Gl. cerebriforme McGee, Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. microaggregatum Koske, Gemma & P.D. Olexia, and Gl. proliferum Dalpe & Declerck. All lack a peridium and contain hyaline to pale yellow spores at least partly overlapping in size. The number of layers in the spore wall, as well as their phenotypic and biochemical properties readily separate Gl. nanolumen from the three fungi mentioned above. Although the wall of spores of Gl. cerebriforme and Gl. microaggregatum also is 2-layered, the inner spore wall layer of Gl. nanolumen is much thicker and more rigid (up to 10.8 µm thick) than the inner spore wall layer of both Gl. cerebriforme (ca. 0.5-1 µm thick, flexible; McGee 1986) and Gl. microaggregatum [0.5-1.2(-2.0) µm thick, flexible to semi-rigid; Błaszkowski, pers. observ.; Koske et al. 1986). The spore wall of Gl. fasciculatum and Gl. proliferum includes three and four layers, respectively (Błaszkowski 2003; Declerck et al. 2000; Walker and Koske 1987). In Gl. fasciculatum, the middle laminate layer stains garnet red (11E8) in Melzer's reagent (Błaszkowski 2003), whereas none of the spore wall layers of Gl. nanolumen reacts in this reagent. Finally, only the laminate spore wall layer of Gl. nanolumen is highly uneven in thickness.
REFERENCES
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone , and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland.http://www.agro.ar.szczecin.pl/~jblaszkowski.
Declerck S., Cranenbrouck S., Dalpé Y., Séguin S., Grandmougin-Ferjani A., Fontaine J., Sancholle M. 2000. Glomus proliferum sp. nov.: a description based on morphological, biochemical, molecular and monoxenic cultivation data. Mycologia 92, 1178-1187.
Koske R. E., Gemma J. N. 1989. Glomus nanolumen (Endogonaceae), a new species from Hawaii. Mycologia 81, 935-938.
Koske R. E., Gemma J. N. 1996. Arbuscular mycorrhizal fungi in Hawaiian sand dunes: Island of Kaua'i. Pacific Sci. 50, 36-45.
Koske R. E., Gemma J. N., Olexia P. D. 1986. Glomus microaggregatum, a new species in the Endogonaceae. Mycotaxon 26, 125-132.
McGee, P. A. 1986. Further sporocarpic species of Glomus (Endogonaceae) from South Australia. Trans. Brit. Mycol. Soc. 87, 123-129.
Walker C., Koske R. E. 1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum redescribed. Mycotaxon 30, 253-262.