Glomus
pubescens
(Sacc. & Ellis) Trappe & Gerd.
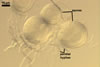
Spores occur in epigeous sporocarps.
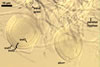
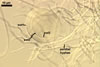
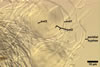
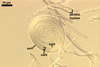
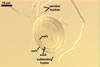
Sporocarps
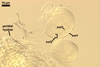
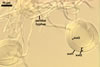
white to light brownish yellow; 0.6-1.0 mm diam; composed of a basal pad of white hyphae, at the tips of which spores origin; spores usually randomly, sometimes radially distributed in the sporocarps, covered with a thin peridium composed of a tangled layer of hyaline, thin-walled hyphae, 1-2 µm wide, from which hyphal tips emerge both singly and in tapered fascicles to form a coarse, sparse to crowded pubescence, 50-100 µm tall. Sand grains sometimes present inside the sporocarps.
SPORES hyaline, globose to subglobose; (25-)31(-36) µm diam; rarely ovoid; 20-24 x 32-36 µm; with one subtending hypha.
SUBCELLULAR
STRUCTURE OF SPORES consists of a spore wall comprising three layers
(swl1-3).
Layer
1,
forming the spore surface, permanent, unit, smooth, hyaline, (0.5-)1.1(-1.5) µm thick.
GERMINATION.
Unknown.
MYCORRHIZAE. All the specimens of Gl. pubescens so far found have been epigeous (Gerdemann and Trappe 1974; Thaxter 1922). According to Gerdemann and Trappe (1974), this fungus is a saprotroph or hyperparasite.
DISTRIBUTION. Thaxter (1922) concluded that Gl. pubescens is uncommon, but probably widely distributed. According to Gerdemann and Trappe (1974), Gl. pubescens is difficult to find because of its small size. To date, this species has been found only twice near the Oregon coast (Gerdemann and Trappe 1974) and once in New Jersey (U.S.A.) and Germany (Thaxter 1922).
NOTES.
The characters of sporocarps of Gl. pubescens presented here are those characterized by Gerdemann and Trappe (1974).
Three characters of spores of Gl. pubescens coming from the Farlow Herbarium (a slide with fragments of a sporocarp mounted in PVLG, Farlow no. 4063, provided by Prof. Rick Koske, Rhode Island University, U.S.A.) and examined by the author of this website differ from those given in the literature. First, the spores characterized here are slightly smaller [(25-)31(-36) µm diam] than those described by Gerdemann and Trappe (1974; 20-48 x 18-45 µm), but somewhat larger than those presented by Thaxter (1922; 18 x 15-25 x 22 µm). Second, the spore wall of spores of the Farlow specimen was markedly thicker [(4.4-)7.6(-10.3) µm thick] than that given by both Gerdemann and Trappe (1974; 3-6 µm thick) and Thaxter (1922; 1.5-2.5 µm thick). Third, all the spores examined were hyaline, and not nearly hyaline to light yellow (Gerdemann and Trappe 1974) or distinctly yellowish (Thaxter 1922).
Apart from Gl. pubescens, other species of Glomus forming sporocarps with a peridium and hyaline to pale yellow spores are Gl. canadense (Thaxt.) Trappe & Gerd., Gl. fulvum (Berk. & Broome) Trappe & Gerd., Gl. microcarpum Tul. & C. Tul., Gl. pallidum I.R. Hall, Gl. pulvinatum (Henn.) Trappe & Gerd., Gl. radiatum (Thaxt.) Trappe & Gerd., Gl. segmentatum Trappe, Spooner & M.H. Ivory, and Gl. vesiculiferum (Thaxt.) Gerd. & Trappe.
Compared with the former fungus producing exceptionally small sporocarps (0.6-1 mm diam; Gerdemann and Trappe 1974), those of all the other species may be much larger (1-2 mm in Gl. pallidum to 1-5 cm in Gl. fulvum; Berch and Fortin 1984; Błaszkowski, pers. observ.; Gerdemann and Trappe 1974; Hall 1977; Tandy 1975; Thaxter 1922; Trappe 1979). Additionally, only the size of spores of Gl. microcarpum is within the size range of spores of Gl. pubescens (Berch and Fortin 1984); spores of the other species are larger.
The most important differences between the species listed above probably reside in the phenotypic and biochemical properties of components of their spore wall. Unfortunately, based on the criteria presently used, the properties have been recognized only in Gl. pallidum and Gl. segmentatum (Błaszkowski, pers. observ.; Oehl et al. 2003). In contrast to the 3-layered spore wall of Gl. pubescens, the spore wall of Gl. pallidum consists of two layers, of which the outer one sloughs with age (vs. it is permanent in Gl. pubescens). The outermost spore wall layer of Gl. segmentatum also is of the type of sloughing layers, although the middle layer and the innermost one of this fungus are similar to those of Gl. pubescens.
REFERENCES
Berch S. M., Fortin J. A. 1984. Some sporocarpic Endogonaceae from eastern Canada. Can. J. Bot. 62, 170-180.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Brit. Mycol. Soc. 68, 341-356.
Oehl F., Wiemken A., Sieverding E. 2003. Glomus aureum, a new sporocarpic arbuscular mycorrhizal fungal species from European grasslands. J. App. Bot. 77, 111-115.
Tandy P. A. 1975. Sporocarpic species of Endogonaceae in Australia. Aust. J. Bot. 23, 849-866.
Thaxter R. 1922. A revision of the Endogonaceae. Proc. Am. Acad. Arts Sci. 57, 291-351.
Trappe J. M. 1979. Glomus segmentatum sp. nov. Trans. Brit. Mycol. Soc. 73, 362-363.