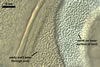
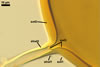
GERMINATION.
A
germ tube emerges from the lumen of the subtending hypha.
MYCORRHIZAE.
In one-species cultures with Plantago lanceolata
L. as the plant host, mycorrhizae of Gl. verruculosum consisted
of arbuscules, vesicles, as well as intra- and extraradical hyphae staining
moderately in 0.1% trypan blue. The arbuscules and vesicles occurred sparsely.
DISTRIBUTION.
Glomus
verruculosum has originally been described from spores isolated from
the root zone of Epilobium
hirsutum L. and Glyceria maxima (Hartm.) Holmb. growing
along the banks of the river Odra near Szczecin (53o26'N, 14o35'E) in north-western
Poland (Błaszkowski
and Tadych 1997). Later, this fungus has also
been found in maritime dunes of the Vistula Bar (54o24'N,
19o30'E; Błaszkowski et al. 2002)
and some cultivated sites of the Western Pomerania (Iwaniuk
and Błaszkowski, pers. observ.).
There
is no information of the occurrence of this fungus in other regions of the
world.
NOTES.
Spores of Gl. verruculosum most resemble those of Gl.
pansihalos S.M. Berch & Koske in colour, size, and in having an ornamentation
formed by warts. However, the warts of the former project inward rather than
outward as in the latter (Berch and Koske 1986). Additionally, the outermost
spore wall layer of Gl. verruculosum is evanescent, whereas
that of Gl. pansihalos is expanding. Finally, the innermost spore
wall layer 3 of Gl. verruculosum usually tightly adheres to the laminate
layer 2, and the third layer of the wall of Gl. pansihalos spores
easily separates from it.
Other species of arbuscular fungi forming
glomoid spores with warts are Gl. callosum Sieverd., Pacispora chimonobambusae
(C.G. Wu & Y.S. Liu) Oehl & Sieverd., and Pac. scintillans
(S.L. Rose & Trappe) Sieverd. & Oehl. In all these
species, the warts occur on the surface of hyaline to white spores of a different
wall structure than that of Gl. verruculosum (Błaszkowski 1988; Rose
and Trappe 1980; Sieverding 1988; Walker, Schüßler 2004; Walker et al. 2004; Wu et al. 1995).
Intact spores of Gl.
verruculosum in early stages of development of warts on the inner surface
of the semirigid spore wall layer 3 may easily be confused with those of Gl.
geosporum (Nicol. & Gerd.)
C. Walker due to similarity in colour, size, and properties of their subtending
hyphae (Błaszkowski, pers. observ.; Miller and Jeffries 1994; Morton 2000;
Walker 1982). However, the third wall layer of both young and mature Gl.
geosporum spores always is smooth, whereas spore wall layer 3 in Gl.
verruculosum is warty. Additionally, the outermost spore wall layer of
Gl. geosporum stains in Melzer's reagent, and that of Gl. verruculosum
does not react in this reagent.
Spores of Gl. verruculosum
also resemble smooth Gl. multiforum
spores (Błaszkowski and Tadych 1997). However, most mature spores of the
latter fungus are pitted (vs. spores with only smooth surface in Gl. verruculosum).
REFERENCES
Berch S. M., Koske R.
E. 1986. Glomus pansihalos: a new species in the Endogonaceae, Zygomycetes.
Mycologia 78, 838-842.
Błaszkowski J. 1988.
Four new species of the Endogonaceae (Zygomycotina) from Poland. Karstenia
27, 37-42.
Błaszkowski J., Tadych
M. 1997. Glomus multiforum and G. verruculosum, two new
species from Poland. Mycologia 89, 804-811.
Błaszkowski J., Adamska
I., Czerniawska B. 2002. Arbuscular mycorrhizal fungi (Glomeromycota) of the
Vistula Bar. Acta Mycol. 37, 39-62.
Miller A. S., Jeffries
P. 1994. Ultrastructural observations and a computer model of the helicoidal
appearance of the spore wall of Glomus geosporum. Mycol. Res. 98,
307-321.
Morton J. B. 2000. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Rose S. L., Trappe J.
M. 1980. Three new endomycorrhizal Glomus spp. associated with actinorrhizal
shrubs. Mycotaxon 10, 413-420.
Sieverding E. 1988.
Two new species of vesicular arbuscular mycorrhizal fungi in the Endogonaceae
from tropical high lands of Africa. Angew. Bot. 62, 373-380.
Walker C. 1982. Species
in the Endogonaceae: a new species (Glomus occultum) and a new combination
(Glomus geosporum). Mycotaxon 15, 49-61.
Walker C., Błaszkowski J., Schwazott D., Schüßler A. 2004. Gerdemannia gen. nov., a genus separated from Glomus, and Gerdemanniaceae fam. nov., a new family in the Diversisporales based on the former Glomus scintillans. Mycol. Res. 108(6), 707-718.
Walker C., Schüßler A. 2004. Nomenclatural clarifications and new taxa in the Glomeromycota. Mycol. Res. 108, 979-982.
Wu C.-G., Liu Y.-S.,
Hwuang Y.-L., Wang Y.-P., Chao C.-C. 1995. Glomales of Taiwan: V. Glomus
chimnobambusae and Entrophospora kentinensis, spp. nov. Mycotaxon
53, 283-294.