 |
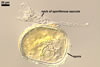
In PVLG |
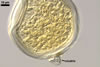
SPORES single in the soil; develop laterally on the neck of a sporiferous saccule; hyaline to white (1A1); globose to subglobose; (80-)94(-115) µm diam; sessile on the neck of a sporiferous saccule.
SUBCELLULAR STRUCTURE OF SPORES composed of a spore wall and two inner germination walls.
Spore wall with two layers (swl1 and 2).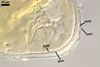 |
 |
 |
 |
 |
In PVLG |
||||
 |
 |
 |
 |
 |
In PVLG |
||||
 |
 |
 |
 |
 |
In PVLG |
In PVLG+Melzer's reagent |
|||
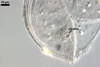
Layer 1 evanescent, hyaline, up to 0.5 µm thick, continuous with the wall of a sporiferous saccule, usually highly deteriorated or completely sloughed in mature spores.
Layer 2 laminate, smooth, hyaline to white (1A1), (1.5-)1.9(-2.3) µm thick, sometimes staining reddish white (7A2) in Melzer’s reagent.
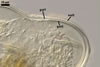
Germination wall 1 with one flexible, hyaline, up to 0.5 µm thick layer (gw1).
Germination wall 2 of two flexible, smooth, hyaline, (0.5-)0.8(-1.0) µm thick, tightly adherent layers (gw2l1 and 2).
None of the germination walls stains in Melzer’s reagent.
GERMINATION ORB. Not found.
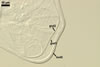
SPORIFEROUS SACCULE hyaline; globose to subglobose; 60-90 µm diam; neck 40-70 µm long, tapering from 10.0-17.5 µm diam at the saccule to 8.0-12.5 µm diam at the point of spore attachment. Saccule collapsing at maturity and usually detached in mature spores.
 |
 |
 |
In PVLG |
||
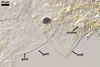
CICATRIX. A slightly raised collar when seen in a cross view, circular, 6.0-9.0 µm diam, when observed in a plane view.
MYCORRHIZAE. In the field, spores of Ac. polonica have occurred among vesicular-arbuscular mycorrhizal roots of Ammophila arenaria (L.) Link, Chamaecyparis lawsoniana Parl., Corynephorus canescens (L.) P. Beauv., Juniperus communis L., and Thuja occidentalis L. Many attempts to produce spores of this fungus in both trap and one-species cultures failed.
DISTRIBUTION. Spores of Ac. polonica has for the first time been found among roots of T. occidentalis growing in Hel (54o36’N, 18o49’E) in northern Poland (Blaszkowski 1988). Later, this fungus has been revealed in maritime dunes of the Hel Peninsula (54o47’N, 18o25’E-54o36’N, 18o49’E) and the Slowinski National Park (54o45’N, 17o26’E; Blaszkowski 1993, 1994; Tadych and Blaszkowski 2000), and in inland dunes of the Bledowska Desert (50o22’N, 19o34’E; Blaszkowski et al. 2002).
There is lack any information of the presence of Ac. polonica in other regions of the world.
NOTES. The only fungus of the genus Acaulospora producing spores similar in size to those of Ac. polonica is Ac. morrowiae Spain & N.C. Schenck. The palest spores of this fungus also are subhyaline. However, its darker spores are pale yellow-brown (Morton 2000), a colour never found in Ac. polonica spores. The subcellular structure of spores of Ac. morrowiae contains seven layers: three in the spore wall and four in two 2-layered germination walls. In contrast, the total number of layers present in spores of Ac. polonica is five. Additionally, the outer layer 1 of the germination wall 2 of Ac. morrowiae spores is ornamented with granular excrescences (“beads”), whereas that of spores of Ac. polonica is smooth. Finally, layer 2 of germination wall 2 of the former species stains red-purple to dark red-purple in Melzer’s reagent, and that of Ac. polonica is nonreactive in this reagent.
Other Acaulospora spp. whose the palest spores are colourless or very light-coloured are Ac. dilatata J.B. Morton and Ac. nicolsonii C. Walker et al. However, while the darkest spores of Ac. polonica are white, those of Ac. delicata and Ac. nicolsonii are pale yellow and pale-yellow-brown, respectively (Morton 1986; Walker et al. 1984). Additionally, even the largest spores of the former species do not reach the lower range of diameter of spores of the two latter fungi. Finally, these fungi differ in subcellular structure of their spores. The structure and phenotypic properties of the spore wall and the germination wall are identical. However, the germination wall 2 of Ac. polonica spores consists of two smooth layers, and that of spores of Ac. delicata is composed of a “beaded” outer layer and a smooth inner layer usually staining light pink to a lightly darker pink (Morton 2000; vs. nonreactive inner layer in Ac. polonica).
The diagnostic description of Ac. nicolsonii indicates this fungus to have only one germination wall (Walker et al. 1984).
REFERENCES
Blaszkowski J. 1988. Four new species of the Endogonaceae (Zygomycotina) from Poland. Karstenia 27, 37-42.
Blaszkowski J. 1993. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Blaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Blaszkowski J., Tadych M., Madej T. 2002. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of the Bledowska Desert, Poland. Acta Soc. Bot. Pol. 71, 71-85.
Morton J. B. 1986. Three new species of Acaulospora (Endogonaceae) from high aluminum, low pH soils in West Virginia. Mycologia 78, 641-648.
Tadych M., Blaszkowski J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National Park, Poland. Mycotaxon 74, 463-483.
Walker C., Reed L. E., Sanders F. E. 1984. Acaulospora nicolsonii, a new endogonaceous species from Great Britain. Trans. Brit. Mycol. Soc. 83, 360-364.