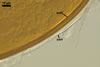
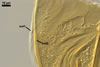
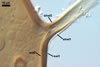
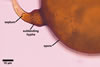
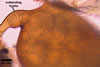
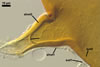
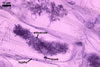
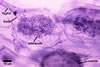
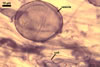
GERMINATION.
A germ tube emerges from the lumen of the subtending
hypha.
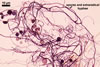
MYCORRHIZAE.
In roots of Zea mays L., mycorrhizae of Gl. coronatum
consisted of arbuscules, vesicles, as well as intra- and abundant extraradical
hyphae staining intensively in 0.1% trypan blue. Arbuscules were numerous
and evenly distributed along the roots examined.
DISTRIBUTION.
Glomus coronatum
has originally been described from spores isolated from under Anacyclus
radiatus Loisel. growing in a maritime sand dune system located near
Follonica, Tuscania, Italy (Giovannetti et al.1991). According to Dodd et
al. (1996), spores of this fungus were earlier found in Israel (Dodd and Krikun
1984), Italy (Giovannetti and Nicolson 1983), Libya (El-Gahmi et al. 1976),
Portugal, Spain, and Australia (Sward et al. 1978). The author of this website
has regularly isolated abundant populations of Gl. coronatum spores
from dunes of the Mediterranean Sea located in Israel (Blaszkowski et al.
2001), Turkey, France, Spain, Portugal, and Morocco, Africa (Blaszkowski,
pers. observ.).
Blaszkowski (1994) reported
the occurrence of Gl. coronatum in Poland and Germany. The spores
of that fungus were similar in colour, size, and had an outer layer of their
wall expanding in lactic acid-based mountants, as those described by Giovannetti
et al. (1991). However, they markedly differed in the shape of the subtending
hypha (cylindrical to flared vs. funnel-shaped in Gl. coronatum).
Reexamination and comparable studies of the two fungi showed that the Polish
and German collections represent an undescribed species.
Recently, Gl. coronatum
has been encountered in cultivated soils of the plain of the upper Rhine River
valley located in France, Germany, and Switzerland (Oehl et al. 003). However,
as the authors of the paper stated, the identification of the spores found
is not valid.
NOTES.
The distinctive characters of Gl. coronatum
are its large and greyish
orange to brownish orange spores and the wide, funnel-shaped subtending hypha.
The fungus most resembling Gl. coronatum is Gl.
mosseae (Nicol. & Gerd.)
Gerd. & Trappe. The two fungi produce spores similar in size with a funnel-shaped
subtending hypha. However, the spores of Gl. mosseae are markedly
lighter and differ in the structure of their wall. The wall of Gl. coronatum
spores consists of two layers, whereas that of spores of Gl. mosseae is
3-layered.
Additionally,
Gl. mosseae is one of the most widely distributed arbuscular fungus
in the world, and both literature data and observations of the author of this
website suggest that Gl. coronatum is restricted to warm regions.
According
to Giovannetti et al. (1991), Gl. coronatum produces spores in sporocarps
surrounded by a peridium. Additionally, the outer spore wall layer of this
fungus was characterized as a structure expanding in lactic acid-based mountants,
as the outer spore wall layer of Gl.
pansihalos S.M. Berch & Koske. All the spores isolated by the author
of this website from field-collected soil samples, as well as from trap and
one-species cultures occurred singly and their outer layer did not expand
either in lactic acid or PVLG.
REFERENCES
Blaszkowski J. 1994.
First record and notes on Glomus coronatum in Poland and Germany.
Mycologia 86, 630-634.
Blaszkowski J., Tadych
M., Madej T., Adamska I., Iwaniuk A. 2001. Arbuscular mycorrhizal fungi (Glomales,
Zygomycota) of Israeli soils. Mat. II Polsko-Izraelskiej Konf. Nauk. nt. „Gospodarowanie
zasobami wodnymi i nawadnianie roslin uprawnych”. Przeglad naukowy Wydz.
Inz. Ksztalt. Srod. 22, 8-27.
Dodd J. C., Krikun J.
1984. Observations on endogonaceous spores in the Negev desert (Israel). Trans.
Br. Mycol. Soc. 82, 536-540.
Dodd J. C., Rosendahl
S., Giovannetti M., Broome A., Lanfranco L., Walker C. 1996. Inter- and intraspecific
variation within the morphologically-similar arbuscular mycorrhizal fungi
Glomus mosseae and Glomus coronatum. New Phytol. 133, 113-122.
El-Giahmi A. A., Nicolson
T. H., Daft M. J. 1976. Endomycorrhiza fungi from Libyan soils. Trans. Br.
Mycol. Soc. 67, 164-169.
Giovannetti M., Nicolson
T. H. 1983. Vesicular-arbuscular my- corrhizas in Italian sand dunes. Trans.
Br. Mycol. Soc. 80, 552-557.
Giovannetti M., Avio
L., Salutini L. 1991. Morphological, cytochemical, and ontogenetic characteristics
of a new species of vesicular-arbuscular mycorrhizal fungus. Canad. J. Bot.
69, 161-167.
Oehl F., Sieverding E.,
Ineichen K., Mader P., Boller T., Wiemken A. 2003. Impact of land use intensity
on the species diversity of arbuscular mycorrhizal fungi in agroecosystems
of Central Europe. Appl. Environ. Microbiol.69, 2816-2824.
Sward R. J., Hallam N.
D., Holland A. A. 1978. Endogone spores in a heathland area of South-Eastern
Australia. Aust. J. Bot. 26, 29-43.