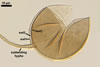
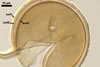
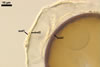
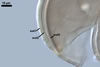
GERMINATION.
Not
observed.
MYCORRHIZAE.
Glomus xanthium was associated in the field with
vesicular-arbuscular mycorrhizae of Xanthium cf. spinosum L. in
Greece, Cenothera
drummondi Hook in Israel, and Ammophila arenaria (L.) Link in
Spain and Turkey (Blaszkowski et al. 2004).
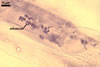
In one-species cultures
with Zea mays L.
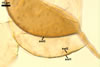
as the plant host, Gl. xanthium formed mycorrhizae with arbuscules,
vesicles, as
well as intra- and extraradical hyphae. Arbuscules were numerous
and generally evenly distributed along the root fragments examined.
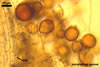
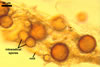
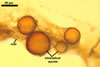
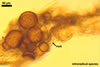
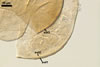
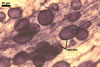
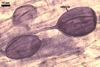
Vesicles occurred numerously and were globose to subglobose, (20-)27(-40) µm
diam, or ellipsoid, 17.5-40.0 x 22.5-120 µm. Intraradical
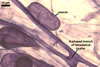
hyphae varied in thickness from (0.9-)5.9(-12.7) µm, grew parallel
to the root axis, and sometimes formed Y- or H-shaped branches and coils,
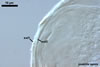
12.5-27.5 x 17.5-55.0 µm. Extraradical hyphae
were abundant, frequently associated with spores, and measured (3.2-)4.7(-7.4) µm
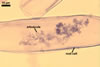
wide. In 0.1% trypan blue, arbuscules stained violet white (16A2) to deep
violet (16D8), vesicles violet white (16A2) to deep violet (16E8), intraradical
hyphae violet white (16A2) to deep violet (16E8), coils violet white (16A2)
to reddish violet (16B6), and extraradical hyphae deep violet (16D8-E8).
PHYLOGENETIC
POSITION. Sequence data and phylogenetic
analyses (Blaszkowski et al. 2004) placed Glomus xanthium in Glomus Group A sensu Schüßler
et al. (2001). Sequences of Gl. xanthium fell into a separate
cluster (Fig. 1) or even formed a separate lineage (Fig. 2) within Glomus Group A, distinct
from all well known species of this group (e. g. Gl. mosseae (Nicol. & Gerd.)
Gerd. & Trappe, Gl.
coronatum Giovann.,
Gl. caledonium (Nicol. & Gerd.) Trappe & Gerd., Gl.
geosporum (Nicol. & Gerd) C. Walker). However, Gl.
xanthium clustered close
to a preliminarily named Glomus sp. ‘Bad Sachsa’ (with
no further correlation to morphological features) from a gypsum slope
of the southern Harz mountains (Germany; Renker et al. 2003, Börstler
et al. unpubl. data), displaying identities between 90 and 94% (Fig.
3).
DISTRIBUTION.
Spores
of Gl. xanthium were for the first time isolated from a trap culture
established with a soil sample collected under X. cf.
spinosum colonizing maritime sand dunes adjacent to Veriko in
northern Greece (22o35’E, 40o08’N; (Blaszkowski et al. 2004). This fungus was not found
in the field-sampled soil. The fungi occurring in the field soil from which Gl.
xanthium inoculum
originated included two unrecognized Glomus spp. and Scutellospora
persica (Koske & C. Walker) C. Walker & F.E. Sanders.
The arbuscular mycorrhizal fungal species associated with Gl. xanthium in
trap cultures were
Gl. clarum Nicol. & N.C. Schenck, Gl. gibbosum Blaszk.,
and an undescribed Glomus sp.
Subsequently, this
fungus was revealed in 15 trap cultures with rhizosphere soils of other
dune sites of the Mediterranean Sea. They were collected
from under
C. drummondi growing near Tel-Aviv (32º4’N, 34º46’E),
Israel, in 1997 (one sample) and 2000 (7 samples), from among roots of
A. arenaria growing near Cape
Salinas (36o19’N, 3o2’E), Majorca,
Spain, in 2001 (2 samples), from under A. arenaria growing near Karabucak-Tuzla
(36o43’N, 34o59’E), Turkey, in 2001 (4 samples), and under A.
arenaria growing near Calambrone (43o35’N, 10o18’E), Italy,
in 2002 (one sample). No study of the composition of arbuscular
mycorrhizal fungal
species in the field-collected soils was undertaken. The arbuscular mycorrhizal
fungi co-occurring with Gl. xanthium in the trap cultures with Israeli
soils were Archaeospora trappei (R.N. Ames & Linderman) J.B.
Morton & D. Redecker, Gl. constrictum Trappe, Gl. claroideum N.C. Schenck & G.S. Sm.,
Gl. coronatum,
Pacispora scintillans (S.L. Rose & Trappe) Sieverd. & Oehl, and Scutellospora pellucida (Nicol. & N.C. Schenck) C. Walker & F.E.
Sanders. The Majorca’s cultures contained Ar. trappei, Gl.
constrictum, Gl. corymbiforme Blaszk., S. calospora (Nicol. & Gerd.)
C. Walker & F.E. Sanders, those from Turkey Gl.
constrictum, Gl.
coronatum, Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl.
aurantium Blaszk. et al., and S.
calospora,
and those from Italy Acaulospora
bireticulata F.M. Rothwell & Trappe, Gl. aurantium, Gl.
microcarpum Tul. & C.
Tul., and S. persica.
NOTES.
Two properties mainly distinguish Gl. xanthium from other Glomus species.
First, spores of the new fungal species tend to form within or tightly
adherent to roots. Second, the spores are relatively
small, with the outermost layer usually thicker than the innermost layer
of the 3-layered spore wall.
The pattern of spore
wall differentiation in Gl.
xanthium is similar to
that of Glomus species investigated to date (Blaszkowski
1997; Blaszkowski and Tadych 1997; Morton 1996; Stürmer and
Morton 1997), with discrete layers formed successively.
When observed under
a dissecting microscope, spores of Gl.
xanthium most
resemble small-spored isolates of Gl.
aggregatum N.C. Schenck & Sm.
emend. Koske and
Gl. intraradices N.C. Schenck & G.S. Sm. These species
produce yellow-coloured spores that frequently occur in both aggregates
tightly associated with roots
and inside them (Schenck and Smith 1982; Stürmer and
Morton 1997).
Using a light microscope,
examination of spores crushed in a mixture of PVLG and Melzer’s reagent
readily separates the three fungi. The spore wall of Gl. xanthium is
composed of two, usually adherent rigid, semi-permanent and permanent layers,
respectively, readily separating from a laminate innermost
layer when crushed. The spore wall of Gl. aggregatum and Gl.
intraradices also consists of three layers, of which two outer ones
usually detach from the innermost laminate layer in crushed spores (Schenck
and Smith 1982; Stürmer and Morton 1997). However, the two
outer spore wall layers of the latter fungi are short-lived and usually
are completely sloughed
in mature
spores (Stürmer and Morton 1997). Additionally,
the outermost wall layer of both Gl. aggregatum and Gl.
intraradices stains
red to purple in
Melzer’s reagent (Stürmer and Morton 1997),
whereas that of Gl. xanthium ramains non-reactive in this reagent.
Finally, the unique
character of Gl. aggregatum is the production of spores inside
their parent spores by internal proliferation (Koske
1985).
Although morphology
placed Gl.
xanthium close to Gl. intraradices, molecular
data did not confirm this estimation. Unfortunately, there is lack of
sequence data
for Gl. aggregatum. Based on available molecular data, Gl.
xanthium can
be considered a member of Glomus Group A sensu Schüßler
at al. (2001). While all
the well known species
of this group
are distinct from Gl. xanthium, Glomus sp. ‘Bad
Sachsa’-sequences were
found to be the closest relatives in the phylogentic analyses.
Firstly detected by Landwehr et al. (2002) at a gypsum slope in the southern
Harz mountains (Germany), similar sequence types were found in further
studies within Germany (Renker et al. 2003, Börstler et al., unpubl.
data). Quite recently, Wubet et al. (2003) detected a Glomus sp.
in Ethiopia colonizing roots
of Prunus africana ITS sequences of this fungus fall into the same sequence cluster like Gl.
xanthium and the original Glomus sp. ‘Bad
Sachsa’ sequence.
Glomus xanthium is
probably adapted to warm soils of southern hemisphere. They have not been
found in any of ca. 3000 soil
samples collected
in different dune and non-dune soils of northern Europe (Blaszkowski
2003). Koske (1987) found temperature to be the main abiotic factor
influencing the structure
of arbuscular fungi of the barrier dunes extending from New Jersey
to Virginia.
According to Pirozynski (1968), temperature is the major factor determining
the distribution and occurrence of fungi in general.
REFERENCES
Blaszkowski J. 1997.
Glomus gibbosum, a new species from Poland. Mycologia 89, 339-345.
Blaszkowski J. 2003.
Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes species deposited in the Department of Plant Pathology, University of
Agriculture in Szczecin, Poland. http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Blaszkowski J., Blanke V., Renker C., Buscot F. 2004. Glomus aurantium and G. xanthium, new species in Glomeromycota. 90, 447-467.
Blaszkowski J., Tadych
M. 1997. Glomus
multiforum and G. verruculosum, two
new species from Poland. Mycologia 89, 804-811.
Landwehr M., Hildebrandt
U., Wilde P., Nawrath K., Tóth T., Biró B.,
Bothe H. 2002. The arbuscular mycorrhizal fungus Glomus geosporum
in European saline, sodic and gypsum soils. Mycorrhiza 12, 199-211.
Koske R. E. 1985. Glomus
aggregatum emended: a distinct taxon in the Glomus fasciculatum complex.
Mycologia 77, 619-630.
Koske R. E. 1987.
Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient.
Mycologia 79, 55-68.
Morton J. B. 1996. Redescription
of Glomus caledonium based on correspondence of spore morphological
characters in type specimens and a living reference
culture. Mycorrhiza 6, 161-166.
Pirozynski K. A. 1968.
Geographical distribution of fungi. In: G. C Ainsworth, A. S Sussman. The
fungi. Academic Press. New York, pp. 487-504.
Renker C., Heinrichs
J., Kaldorf M., Buscot F. 2003. Combining nested PCR and restriction digest
of the internal transcribed spacer region to
characterize arbuscular mycorrhizal fungi on roots from the field. Mycorrhiza
13,
191-198.
Schenck N. C., Smith
G. S. 1982. Additional new and unreported species of mycorrhizal fungi
(Endogonaceae) from Florida. Mycologia 74, 77-92.
Schüßler
A., Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota:
phylogeny and evolution. Mycol. Res. 105, 1413-1421.
Stürmer S. L.,
Morton J. B. 1997. Developmental patterns defining morphological characters
in spores
of four species in Glomus. Mycologia 89, 72-81.
Wubet T., Weiß M.,
Kottke I., Teketay D., Oberwinkler F. 2003. Molecular diversity of arbuscular
mycorrhizal fungi in Prunus africana, an endangered
medicinal tree species in dry Afromontane forests of Ethiopia. New Phytol.
161, 517-528.