Acaulospora lacunosa J.B. Morton
 |
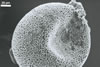
SPORES single in the soil; develop laterally on the neck of a sporiferous saccule; yellow (4A6) to reddish yellow (4A8); globose to subglobose; (85-)110(-140) µm diam; rarely ovoid; 90-110 x 130-140 µm.
SUBCELLULAR STRUCTURE OF SPORES consists of a spore wall and two inner germination walls.
Spore wall composed of two layers (swl1-2).
SEM |
In PVLG |
|||||
 |
 |
 |
 |
 |
 |
 |
In PVLG |
In PVLG+Melzer's reagent |
|||||
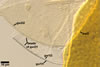
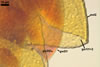
Layer 1 evanescent, hyaline, 0.5-0.8 µm thick, continuous with the wall of a sporiferous saccule, usually completely sloughed in mature spores.
Layer 2 laminate, yellow (4A6) to reddish yellow (4A8), (2.2-)3.6(-5.1) µm thick, ornamented with round, prolate, most frequently irregularly shaped and distributed pits, 0.5-1.2 x 0.6-3.4 µm, when seen in a plan view, 1.3-1.7 µm deep, when observed in a cross-sectional view.
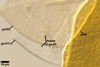
Germination wall 1 consists of two hyaline, semiflexible layers (gw1l1 and 2), each ca. (0.5-)0.8(-1.0) µm thick; these layers usually separate from each other in crushed spores.
Germination wall 2 composed of two adherent layers (gw2l1 and 2).
Layer 1 flexible, hyaline, 0.5-1.7 µm thick, covered with small, <0.5 µm diam, granules sometimes scattering in crushed spores.
Layer 2 plastic, hyaline, 2-18 µm thick in PVLG, (0.5-)0.8(-1.2) µm thick and dark purplish red (13E8) in Melzer’s reagent.
GERMINATION ORB. Not found.
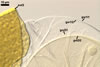
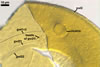
SPORIFEROUS SACCULE hyaline to light yellow (3A5); globose to subglobose; 80-90 µm diam; neck 60-90 µm long, 13.0-15.0 µm wide at the saccule, tapering to 10.0-12.0 µm wide at the spore attachment. Saccule wall consists of a hyaline, smooth, 0.8-1.2 µm thick layer. Saccule usually collapses or falls off in mature spores.
 |
In
PVLG |
CICATRIX. A slightly raised collar, circular, 10-12.5 µm diam when seen in a plane view.
MYCORRHIZAE. In Poland, Ac. lacunosa has been associated in the field with vesicular-arbuscular mycorrhizal roots of many plant species (Błaszkowski 1990, 1993a, b, 1994; Tadych and Błaszkowski 2000a, b; Błaszkowski, pers. observ.). According to Morton (2000), this fungus formed vesicular-arbuscular mycorrhizae staining variably, depending on age of the mycorrhizae and host plant.
DISTRIBUTION. Acaulospora lacunosa has commonly occurred in sandy dune soils of the Baltic Sea coast (Błaszkowski 1990, 1993b, 1994; Tadych and Błaszkowski 2000a) and has frequently been revealed in cultivated and other uncultivated soils of Poland (Błaszkowski 1993a; Błaszkowski, pers. observ.; Tadych and Błaszkowski 2000b).
Acaulospora lacunosa has originally been recovered from among the roots of Andropogon virginicus L. in West Virginia (Morton 1986). According to Koske and Gemma (1997), Ac. lacunosa is a relatively common inhabitant of dunes of the U. S. Atlantic coast from Massachusetts to Virginia.
NOTES. The uniqueness of of Ac. lacunosa results from the distinctive ornamentation of its spores. Pitted spores are also produced by Ac. cavernata Blaszk. Ac. foveata Trappe & Janos, Ac. paulinae Blaszk., Ac. scrobiculata Trappe, and Ac. undulata Sieverd. (Błaszkowski 1988, 1989; Janos and Trappe 1982; Sieverding 1988; Trappe 1977). However, the pits of Ac. lacunosa spores are more both irregularly-shaped and scattered. Additionally, spores of Ac. cavernata and Ac. foveata are darker-coloured and larger, and those of Ac. paulinae and Ac. undulata are smaller and lighter. Compared with spores of Ac. lacunosa, those produced by Ac. scrobiculata are larger and lighter-coloured.
REFERENCES
Błaszkowski J. 1988. Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland. Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Błaszkowski J. 1989. Acaulospora cavernata (Endogonaceae) - a new species from Poland with pitted spores. Crypt. Bot. 1, 204-207.
Błaszkowski J. 1990. Polish Endogonaceae. VI. Acaulospora lacunosa. Crypt. Bot. 2, 20-24.
Błaszkowski J. 1993a. Comparative studies of the occurrence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28, 93-140.
Błaszkowski J. 1993b. The occurrence of arbuscular fungi and mycorrhizae (Glomales) in plant communities of maritime dunes and shores of Poland. Bull. Pol. Ac. Sci. Biol. Sci. 41, 377-392.
Błaszkowski J. 1994. Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland. Mycorrhiza 5, 71-88.
Janos D. P., Trappe J. M. 1982. Two new Acaulospora species from tropical America. Mycotaxon 15, 515-522.
Koske R. E., Gemma J. N. 1997. Mycorrhizae and succession in plantings of beachgrass in sand dunes. Amer. J. Bot. 84, 118-130.
Morton J. B. 1986. Three new species of Acaulospora (Endogonaceae) from high aluminum, low pH soils in West Virginia. Mycologia 78, 641-648.
Sieverding E. 1988. Two new species of vesicular arbuscular mycorrhizal fungi in the Endogonaceae from tropical high lands of Africa. Angew. Bot. 62, 373-380.
Tadych M., Błaszkowski J. 2000a. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National Park, Poland. Mycotaxon 74, 463-483.
Tadych M., Błaszkowski J. 2000b. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola Forests. Acta Mycol. 35, 3-23.
Trappe J. W. 1977. Three new Endogonaceae: Glomus constrictus, Sclerocystis clavispora, and Acaulospora scrobiculata. Mycotaxon 6, 359-366.