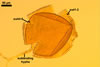
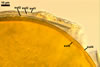
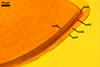
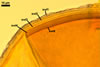
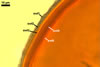
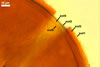
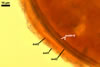
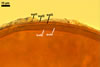
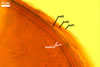
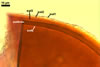
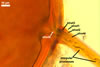
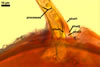
Layer
1, forming the spore surface, semi-permanent, of a roughened surface, hyaline, <1.0 µm thick; more or less degraded and then granular in most mature spores, but always present.
Layer
2 laminate, hyaline, 1-3 µm thick in water, swelling up to 10-20(-30) µm in lactic acid-based mountants, forming an irregular flexible halo.
Layer 3
finely laminate, hyaline to pale yellow (2A3) to pastel yellow (2A4) with a greenish tint, 1.0-4.5 µm thick, rarely separates from layer 2.
Layer 4
laminate, light orange (5A5) to dark orange (5A8), usually tightly adherent to layer 3.
Layer 5
semi-flexible, consists of at least two sublayers (laminae), concolorous with layer 4, 0.5-1.0 µm thick.
In Melzer's reagent, only layer 2 stains pale red (9A3) to pastel red (9A4).
GERMINATION.
Not determined to date.
MYCORRHIZAE.
According to Oehl et al. (2002), in one-species cultures, Gl. caesaris formed vesicular-arbuscular mycorrhizae in roots of Hieracium pilosella L.
DISTRIBUTION. The type of Gl. caesaris has been designated from spores extracted from a one-species culture of this fungus, in which H. pilosella was the host plant (Oehl et al. 2002). The only so far known site of the natural occurrence of Gl. caesaris is a vineyard located at the Vogelsang Pass near Vogtsburg, Kaiserstuhl area, Germany.
NOTES.
The morphological and biochemical properties of spores and mycorrhizae of Gl. caesaris presented above were prepared based on the original description of this fungus (Oehl et al. 2002) and observations of its spores loaned from Dr. F. Oehl, Institute of Botany, University of Basel, Switzerland.
The unique structure of Gl. caesaris is its second spore wall layer, which swells in lactic acid-based mountants and is reactive in Melzer's reagent. The only other species of the phylum Glomeromycota having a spore wall layer of similar morphological and biochemical properties and positioned identically is Scutellospora biornata Spain, Sieverd. & Toro (Spain et al. 1989).
Spores of Gl. caesaris deceptively reminiscent of those of Gl. caledonium (Nicol. & Gerd.) Trappe & Gerd., when observed under both a dissecting and a compound microscope. They are similar in colour and overlap in a wide range of their size (Błaszkowski 2003; Morton 1996, 2002). However, the fungi markedly differ mainly in the properties of all the three layers lying over the laminate structural layer of their spore wall. Of them, in Gl. caesaris, the two inner layers 2 and 3 are laminate, and the layer forming the spore surface is granular and long-lived. In contrast, in Gl. caledonium, the two inner layers differ morphologically and none is laminated, and the outermost layer quickly sloughs and rarely occurs in even young spores. All the layers are colourless, whereas layer 3 in mature spores of Gl. caesaris is yellow-coloured. The unique character of the layer directly covering the structural laminate wall layer of spores of Gl. caledonium is its fragility and brilliance in polarized light. This layer disintegrates into small fragments and, thereby, resembles a hardened glass. Moreover, the spore wall of Gl. caledonium does not possess the spore wall layer 5 of Gl. caesaris. Finally, in the former species, the spore wall layer staining in Melzer's reagent is the mucilagenous outermost one, whereas the reactive layer in this reagent in the latter species is the second laminate layer.
REFERENCES
Agrios G. N. 1988. Plant pathology, 3rd edition, Academic Press, INC. San Diego, New York, Berkeley, Boston, London, Sydney, Tokyo, Toronto.
Morton J. M. 1996. Redescription of Glomus caledonium based on correspondence of spore morphological characters in type specimens and a living reference culture. Mycorrhiza 6, 161-166.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Oehl F., Wiemken A., Sieverding E. 2002. Glomus caesaris, a new arbuscular mycorrhizal fungus from the Kaiserstuhl in Germany. Mycotaxon 84, 379-385.
Spain J. L., Sieverding E., Toro S. 1989. Scutellospora biornata: a new species in the Endogonaceae from the Llanos Orientales of Colombia. Mycotaxon 35, 219-227.