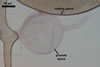
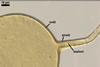
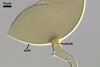
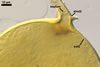
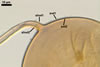
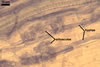
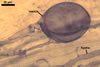
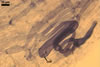
GERMINATION.
A
germ tube emerges from the lumen of the subtending hypha.
MYCORRHIZAE. The mycorrhizae formed in one-species cultures of this fungus
with Plantago lanceolata L. as the plant host consisted of
arbuscules, vesicles, as well as intra- and extraradical hyphae. Arbuscules
were irregularly distributed and had fine branches. Vesicles measured 33.9-57.2
x 51.3-137.3 µm and were usually highly scattered along roots. Intraradical
hyphae were 1.4-8.9 µm wide and grew parallel to the root axis. They frequently
had thickenings or short outgrowths. These hyphae sometimes formed coils, 14.2-27.2
x 26.3-60.0 µm and Y-shaped branches. Extraradical hyphae were 1.4-4.1
µm wide. In 0.1% trypan blue, arbuscules stained violet white (18A2) to
greyish violet (18C4), vesicles pastel violet (18A4) to deep violet (18E8),
intraradical hyphae violet white (18A2) to dull violet (18D4), coils pale violet
(18A3) to greyish violet (18D7), and extraradical hyphae pastel violet (18A4)
to greyish violet (18C5).
DISTRIBUTION.
The author of this website
has so far found
spores of Gl. deserticola in 199 field-collected soil samples. Of
them, 197 came from cultivated and uncultivated soils of Poland, and two were
collected in natural sites of the Czech Republic and Lithuania. All the soils
have been sampled from under 45 species in 12 plant families.
The literature data showing
the origin of arbuscular mycorrhizal fungi used in experiments and their geographical
occurrence indicate that Gl. deserticola has been found only in the
U.S.A. (Augé 1989; Bloss and Walker 1987; Paulitz and Menge 1986; Sylvia
1986; Sylvia and Will 1988; Trappe et al. 1984; Will and Sylvia 1990), Spain
(Arines and Vilarino 1991), Poland (Błaszkowski 1990, 1993a, b, 1994; Tadych,
Błaszkowski 2000), and India (Ragupathy and Mahadevan 1993). However, the
diversity of climatic and soil conditions of these countries, as well as the
data on the fungus presented here suggest it to have a worldwide distribution.
Additionally, Gl. deserticola has probably many times been mistakenly
identified as Gl. fasciculatum (Walker and Koske 1987; Trappe et
al. 1984), one of the most frequently reported arbuscular mycorrhizal fungus
from soil surveys and most often cited as used in studies of plant growth
responses (Walker 1985).
NOTES.
When observed under a dissecting microscope, the species of the genus Glomus
most resembling Gl. deserticola are Gl.
aggregatum N.C. Schenck & G.S. Sm. emend. Koske, Gl.
fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske,
Gl. intraradices N.C. Schenck & G.S. Sm., and Gl. hoi S.M. Berch & Trappe (Berch and Trappe 1985; Koske 1985; Stürmer and Morton 1997;
Walker and Koske 1987). Spores of all the fungi occur singly or in aggregates
in the soil, are yellow-coloured, and have a similar shape and size range.
Glomus pustulatum Koske et
al. and Gl. trimurales Koske
& Halvorson also produce spores similar in colour and size, but they are
formed only singly in the soil (Koske et al. 1986; Koske and Halvorson 1989).
Glomus deserticola
differs from the species listed above in number, as well as in phenotypical
and staining properties of its spore wall layers. They are most evident when
spores crushed in a mixture of PVLG and Melzer’s reagent are examined under
a compound microscope.
While the spore wall
of Gl. deserticola consists of two layers: a mucilaginous layer adherent
to a laminate layer, that of spores of Gl. aggregatum, Gl. fasciculatum,
and Gl. intraradices contains three layers. Glomus deserticola
lacks the flexible innermost layer of Gl. fasciculatum (Walker and
Koske 1987) and the semiflexible middle layer of Gl. aggregatum and
Gl. intraradices (Błaszkowski, pers. observ.; Stürmer and Morton
1997). Additionally, the outermost wall layer of Gl. fasciculatum
spores is permanent and the innermost spore wall layer of Gl. intraradices
consists of readily separating sublayers (laminae). In contrast, the outer
layer of Gl. deserticola spores sloughs with age, and their inner
laminate layer consists of tightly adherent laminae. Finally, only the outermost
mucilaginous layer of spores of Gl. deserticola, Gl.
aggregatum and Gl. intraradices reacts in Melzer’s reagent,
whereas all three layers of Gl. fasciculatum spores stain in this
reagent. Additionally, the unique property of Gl. aggregatum
is the production of inner spores by internal proliferation (Koske 1985; Błaszkowski
1991).
Although Gl. hoi
is described to produce two-layered spores as does Gl. deserticola,
the outer layer of the former fungus is thicker and coloured, and that of
the latter species is thinner and colourless (Berch and Trappe 1985; Morton
2000).
The spores of Gl.
pustulatum and Gl. trimurales
have a wall composed of three permanent layers, of which none stains in
Melzer’s reagent (Błaszkowski, pers. observ.; Koske and Halvorson 1989;
Koske et al. 1986; Morton 2000; vs. two layers with an outer layer staining
reddish white to bluish red in this reagent in Gl. deserticola).
REFERENCES
Arines J., Vilarino A.
1991. Growth, micronutrient content and vesicular-arbuscular fungi infection
of herbaceous plants on lignite mine spoils: a greenhouse pot experiment.
Plant and Soil 135, 269-273.
Augé R. M. 1989.
Do VA mycorrhizae enhance transpiration by affecting host phosphorous content?
J. Plant nutrition 12, 743-753.
Berch S. M., Trappe J.
M. 1985. A new species of Endogonaceae, Glomus hoi. Mycologia 77,
654-657.
Bloss H. E., Walker
C. 1987. Some endogonaceous mycorrhizal fungi of the Santa Catalina Mountains
in Arizona. Mycologia 79, 649-654.
Błaszkowski J. 1990.
Polish Endogonaceae IV. Gigaspora gigantea, Glomus deserticola,
and Glomus globiferum. Acta Mycol. 26, 3-16.
Błaszkowski J. 1991.
Polish Endogonaceae. IX. Glomus aggregatum with spores forming an
evanescent outermost wall. Crypt. Bot. 2/3, 130-135.
Błaszkowski J. 1993a.
Comparative studies of the occurrence of arbuscular fungi and mycorrhizae
(Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol. 28,
93-140.
Błaszkowski J. 1993b.
The occurrence of arbuscular fungi and mycorrhizae (Glomales) in plant communities
of maritime dunes and shores of Poland. Bull. Pol. Ac. Sci. Biol. Sci. 41,
377-392.
Błaszkowski J. 1994.
Arbuscular fungi and mycorrhizae (Glomales) of the Hel Peninsula, Poland.
Mycorrhiza 5, 71-88.
Koske
R. E. 1985. Glomus aggregatum emended: A distinct taxon in the Glomus
fasciculatum complex. Mycologia 77, 619-630.
Koske R. E., Halvorson
W. L. 1989. Scutellospora arenicola and Glomus trimurales:
two new species in the Endogonaceae. Mycologia 81, 927-933.
Koske R. E., Friese
C., Walker C., Dalpé Y. 1986. Glomus pustulatum: A new species
in the Endogonaceae. Mycotaxon 26, 143-149.
Morton J. B. 2000. International
Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi.
West Virginia University.
Paulitz T. C., Menge
J. A. 1986. The effects of a mycoparasite on the mycorrhizal fungus, Glomus
desertiola. Phytopathol. 76, 351-354.
Ragupathy S., Mahadevan
A. 1993. Distribution of vesicular-arbuscular mycorrhizae in the plants and
rhizosphere soils of the tropical plains, Tamil Nadu, India. Mycorrhiza 3,
123-136.
Stürmer S. L., Morton
J. B. 1997. Developmental patterns defining morphological characters in spores
of four species in Glomus. Mycologia 89, 72-81.
Sylvia D. M. 1986. Spatial
and temporal distribution of ve- sicular-arbuscular mycorrhizal fungi associated
with Uniola paniculata in Florida foredunes. Mycologia 78, 728-734.
Sylvia D. M., Will M.
E. 1988. Establishment of vesicular-arbuscular mycorrhizal fungi and other
microorganisms on a beach replenishment site in Florida. Appl. Environm. Microbiol.
54, 348-352.
Tadych M., Błaszkowski
J. 2000. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National
Park, Poland. Mycotaxon 74, 463-483.
Trappe J. W., Bloss
E., Menge J. 1984. Glomus deserticola sp. nov. Mycotaxon 20, 123-127.
Walker C. 1985. Taxonomy
of the Endogonaceae. In: 6th North American Conference on Mycorrhizae. Proc.
Ed., R. Molina. Forest Research Laboratory, Corvalis, Oregon, 193-198.
Walker C., Koske R. E.
1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum
redescribed. Mycotaxon 30, 253-262.
Will M. E., Sylvia D.
M. 1990. Interaction of rhizosphere bacteria, fertilizer, and vesicular-arbuscular
mycorrhizal fungi with sea oats. Appl. Environm. Microbiol. 56, 2073-2079.