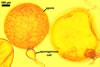
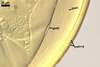
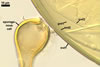
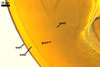
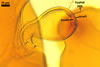
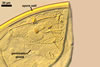
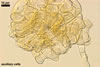
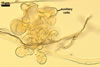
NOTES. The distinctive morphological characters of S. fulgida are its light-coloured and smooth spores having only one inner germinal wall. The last property keys this fungus into a monophyletic group still comprising S. castanea, S. coralloidea, S. gregaria, S. persica, and S. verrucosa.
Four spore characters readily separate the species listed above. First, spores of S. fulgida are much lighter-coloured than those of the other species compared here (cream to light orange in S. fulgida vs. from pale straw to orange brown in S. verrucosa to red brown to dark brown in S. gregaria; Morton 1995, 2002). Second, in contrast to the smooth spores of S. castanea (Walker et al. 1993) and S. fulgida, the upper spore surface of the other species is ornamented with warts (Morton 1995, 2002). However, S. fulgida and S. castanea markedly differ in colour and size of spores. The darkest spores of the former fungus are of a yellow shade, and mature spores of the latter species are brown (Walker et al. 1993). Third, although the lower size range of globose spores of S. fulgida and S. castanea overlaps, the largest spores of S. fulgida (280 µm diam) are much smaller than the greatest spores of S. castanea (up to 372 µm diam; Walker et al. 1993). Spores of the other species discussed here may also attain a much higher size than those of S. fulgida (384 µm diam in S. persica to 480 µm diam in S. gregaria; Morton 1995). Fourth, similarly as in S. castanea, the warts ornamenting the germination shield of S. fulgida spores are much lower and less densely dispersed on its upper surface compared with those ornamenting the germination shield of the other species. This also causes the germination shields of the former two species to be relatively more flexible, as Morton (1995) concluded.
REFERENCES
De Souza F. A., Declerck S., Smit E., Kowalchuk G. A. 2005. Morphological, ontogenetic and molecular characterization of Scutellospora reticulata (Glomeromycota). Mycol. Res. 109, 697-706.
Gai J. P., Christie P., Feng G., Li X. L. 2006. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China : a review. Mycorrhiza 16, 229-239.
Koske R. E. 1987. Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia 79, 55-68.
Koske R. E., Walker C. 1986. Species of Scutellospora (Endogonaceae) with smooth-walled spores from maritime sand dunes: two new species and a redescription of the spores of Scutellospora pellucida and Scutellospora calospora. Mycotaxon 27, 219-235.
Morton J. M. 1995. Taxonomic and phylogenetic divergence among five Scutellospora species based on comparative developmental sequences. Mycologia 87, 127-137.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Schalamuk S., Velazquez S., Chidichimo H., Cabello M. 2006. Fungal spore diversity of arbuscular mycorrhizal fungi associated with spring wheat: effect of tillage. Mycologia 98, 16-22.
Sylvia D. M., Will M. E. 1988. Establishment of vesicular-arbuscular mycorrhizal fungi and other microorganisms on a beach replenishment site in Florida. Appl. Environ. Microbiol. 54, 348-352.
Walker C., Gianinazzi-Pearson V., Marion-Espinasse H. 1993. Scutellospora castanea, a newly described arbuscular mycorrhizal fungus. Cryptog. Mycol. 14, 279-286.