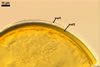
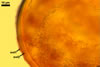
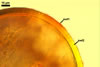
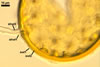
GERMINATION.
None of the spores examined by the author of this website germinated. According to Stürmer and Morton (1997), spores of Gl. etunicatum germinate through a germ tube emerging from the lumen of the subtending hypha.
MYCORRHIZAE. According to Morton (2002), in one-species cultures with Sorghum sudanense (Piper) Staph, S. vulgare Pers. and Zea mays L., mycorrhizae of Gl. etunicatum consisted of arbuscules, vesicles, as well intra- and extraradical hyphae. All the structures stained intensively in trypan blue.
DISTRIBUTION. The type of Gl. etunicatum has been selected from spores isolated from under Allium cepa L. cultivated in a pot culture containing a prairie sand of Illinois, U.S.A. (Baker and Gerdemann 1977). Literature data suggest Gl. etunicatum to be one of the most commonly occurring arbuscular fungi in the world. Apart from many other its findings in different cultivated and uncultivated sites of the U.S.A. (e. g., Bentivenga and Hetrick 1992; Koske and Tews 1987; Schenck and Smith 1981), Gl. etunicatum has also been recorded in, e. g., Canada (Dalpé et al. 1986), Mexico (Estrada-Tores et al. 1992), Brazil (Grandi and Trufem 1991), France, Switzerland and Germany (Oehl et al. 2005), Spain (Diaz et al. 1992), Poland (Błaszkowski 1990), Africa (Musoko et al. 1994; Stutz et al. 2000), Israel (Błaszkowski et al. 2001), China and Taiwan (Gai et al., in press).
The specimens of Gl. etunicatum examined by the author of this website came from Bolivia and India. Their morphological and biochemical properties fully fitted those characterized by Becker and Gerdemann (1977) and Stürmer and Morton (1997), but slightly differed from the properties of spores found in Poland (Błaszkowski 1990). Polish specimens of this fungus came from the field and their sloughing outer spore wall layer did not stain in Melzer's reagent. Later studies of the occurrence of arbuscular fungi in many regions of Poland and many other countries of Europe conducted based on spores extracted from pot trap cultures never revealed this species (Błaszkowski, pers. observ.). However, the fungus frequently encountered and highly resembling Gl. etunicatum was Gl. claroideum.
NOTES.
When seen under a dissecting microscope, spores of Gl. etunicatum may easily be confused with those of Gl. arenarium Blaszk. et al., Gl. claroideum N.C. Schenck & G.S. Sm., Gl. clarum Nicol. & N.C. Schenck, Gl. geosporum (Nicol. & Gerd.) C. Walker, Gl. lamellosum Dalpé et al., Gl. luteum L.J. Kenn. et al., Gl. verruculosum Blaszk., and Gl. versiforme (P. Karsten) S.M. Berch. All the fungi produce yellow-coloured spores of a more or less overlapping range of their size. However, of them, only Gl. claroideum, Gl. clarum, Gl. geosporum, and Gl. luteum have a spore wall with a mucilaginous outer layer of biochemical properties similar to those of wall layer 1 of spores of Gl. etunicatum (Błaszkowski 2003; Stürmer and Morton 1997; Kennedy et al. 1999). These fungi separate the number of the other layers in the wall of their spores. Whereas the wall of spores of Gl. etunicatum is 2-layered, that of Gl. clarum and Gl. geosporum comprises three layers, and the spore wall of Gl. claroideum and Gl. luteum consists of four layers. Spores of Gl. etunicatum lack the third laminate wall layer of Gl. clarum and the third semi-rigid wall layer of Gl. geosporum, as well as the second semi-flexible wall layer and the forth flexible wall layer of Gl. claroideum and Gl. luteum.
Glomus versiforme forms spores with a 2-layered wall (Błaszkowski 2003;
Błaszkowski et al. 2003). However, in contrast to the sloughing with age and staining in Melzer's reagent outer layer of Gl. etunicatum, the outer layer of spores of Gl. versiforme is semi-permanent and does not react in this reagent.
Glomus etunicatum diverges from Gl. arenarium, Gl. lamellosum, and Gl. verruculosum in respect of both the number of layers in their spore wall and the reactivity of the outermost spore wall layer in Melzer's reagent. All the latter species produce spores with their 3-layered wall, of which none reacts in Melzer's reagent (Błaszkowski 2003; Błaszkowski and Tadych 1997; Błaszkowski et al. 2001; Błaszkowski et al. 2002) .
Results of molecular investigations have placed Gl. etunicatum in two of the three separated groups of the genus Glomus, i. e., Glomus group B and Glomus group C in the family Glomeraceae Piroz. & Dalpé of the order Glomerales J.B. Morton & Benny (Schüßler et al. 2001). In Glomus group B, Gl. etunicatum is accompanied by Gl. claroideum, Gl clarum, Gl. lamellosum and Gl. luteum, species whose the close morphological similarity was presented above. In Glomus group C, the species co-occurring with Gl. etunicatum are Gl. spurum C.M. Pfeiff. et al. emend. L.J. Kenn. et al. and Gl. versiforme. Glomus spurcum has recently been transferred to the genus Diversispora C. Walker & Schuessler and renamed D. spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler (Walker and Schüßler 2004). The reason of the location of different isolates of Gl. etunicatum in two distinct clades has not been clarified to date.
REFERENCES
Becker W. N., Gerdemann J. W. 1977. Glomus etunicatus sp. nov. Mycotaxon 6, 29-32.
Bentivenga S. P., Hetrick B. A. D. 1992. Seasonal and temperature effects on mycorrhizal activity and dependence of cool- and warm-season tallgrass prairie grasses. Can. J. Bot. 70, 1596-1602.
Błaszkowski J. 1990. Polish Endogonaceae 2. Acaulospora rugosa, Glomus aggregatum, Glomus etunicatum, Glomus fasciculatum and Glomus occultum. Karstenia 30, 1-13.
Błaszkowski J., Adamska I., Czerniawska B. 2003. Acaulospora scrobiculata and Glomus versiforme (Glomeromycota), arbuscular fungi newly and second time, respectively, found in Poland. Acta Mycol. 38 (1/2), 31-42.
Błaszkowski J., Adamska I., Madej T. 2002. Glomus lamellosum (Glomales, Zygomycota), an arbuscular mycorrhizal fungal species new for Poland and Europe. Mycotaxon 81, 281-292.
Błaszkowski J., Tadych T. 1997. Glomus multiforum and G. verruculosum, two new species from Poland. Mycologia 89, 804-811.
Błaszkowski J., Tadych M., Madej T. 2001. Glomus arenarium, a new species in Glomales (Zygomycetes). Acta Soc. Bot. Pol. 70, 97-101.
Błaszkowski J., Tadych M., Madej T., Adamska I., Iwaniuk A. 2000. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of Israeli soils. Mat. II Polsko-Izaelskiej Konf. Nauk. nt. "Gospodarowanie zasobami wodnymi i nawadnianie roslin uprawnych". Przeglad naukowy Wydz. Inz. Ksztalt. Srod. 22, 8-27.
Dalpé Y., Granger R. L., Furlan V. 1986. Abondance relative et diversite des Endogonacees dans un sol der verger du Quebec. Can. J. Bot. 64, 912-917.
Diaz G., Roland A., Albaladejo J. 1992. Influencia del tipo de suelo sobre las pautas de colonizacion y eficiencia en la simbiosis micorricica de seis especies de Glomus. Crypt. Mycol. 13, 47-56.
Estrada-Torres A., Varela L., Hernandez-Cuevas L., Cavito M. E. 1992. Algunos hongos micorrizicos arbusculares del estado de Tlaxcala, México. Rev. Mex. Mic. 8, 85-110.
Gai J. P., Christie P., Feng G., Li X. L. 2005. Twenty years of research on biodiversity and distribution of arbuscular mycorrhizal fungi in China : a review. Mycorrhiza (in press).
Grandi R. A. P., Trufem S. F. B. 1991. Fungos micorrizos vesiculo-arbusculares em Marantaceae cultivadas no Instituto de Botanica, Sao Paulo, SP. Revista brasil. Bot. 14, 89-95.
Kennedy L. J., Stutz J. C., Morton J. B. 1999. Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91, 1083-1093.
Koske R. E., Tews L. L. 1987. Vesicular-arbuscular mycorrhizal fungi of Wisconsin sandy soils. Mycologia 79, 901-905.
Morton J. B. 2002. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University: http://www.invam.caf.wvu.edu/.
Musoko M., Last F. T., Mason P. A. 1994. Popultions of spores of vesicular-arbuscular mycorrhizal fungi in undisturbed soils of secondary semideciduous moist tropical forest in Cameroon. Forest Ecol. Manag. 63, 359-377.
Oehl F., Sieverding E., Ineichen K., Ris E.-A., Boller T., Wiemken A. 2005. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 165, 273-283.
Schüßler A., Gehrig H., Schwarzott D., Walker C. 2001. Analysis of partial Glomales SSU rRNA gene sequences: implications for primer design and phylogeny. Mycol Res. 105, 5-15.
Schenck N. C., Smith G. S. 1981. Distribution and occurrence of vesicular-arbuscular mycorrhizal fungi on Florida agricultural crops. Soil and Crop Sci. Soc. Florida 40, 171-175.
Stutz J. C., Copeman R., Martin C. A., Morton J. B. 2000. Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southeastern North America and Namibia, Africa. Can. J. Bot. 78, 237-245.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Walker C., Schüßler A. 2004. Nomenclatural clarifications and new taxa in the Glomeromycota. Mycol. Res. 108, 979-982.