MYCELIUM of two types: primary and secondary, formed on stems, inflorescences, and leaves.
Primary mycelium amphigenous, thin to dense, at first in patches, later effuse, white when young, ochraceous to rusty brown when old.
Secondary mycelium composed of bristle-like, at first hyaline, later greyish to rusty brown, aseptate, straight to curved, falcate, thick-walled, ca. 200-400 x 4-7 µm hyphae; this mycelium usually forms a dense felt on the host leaves and stems.
in lactic acid
|
in lactic acid + trypan blue |
|||||
HAUSTORIA (h) hyaline, digitate.
 |
HYPHAE flexuous, septate, hyaline to rusty brown, with cells (35-)43(-53) x 3.5-5.5 µm, with single or in pairs, nipple-shaped, 3.5-7 µm wide, appressoria (a).
FOOT CELLS (fc) 20-40 x 5-7 µm, with a bulbous swelling, ca. 10-15 µm wide, followed by shorter cells, ca. 12.5-25 µm long.
CONIDIA (c) in chains, ellipsoid to lemon-shaped, hyaline, (20-)24-35(-45) x (8-)12-16(-20) µm, rarely wider at one end or slightly constricted at the center.
GERMINATION OF CONIDIA by formation of germ tubes at the end or side of the conidium; germ tubes straight or flexuous, ca. 12-50 x 2.5-4 µm, terminating in an unlobed appressorium.
CLEISTOTHECIA subscattered to gregarious, at first globose, later concave, yellowish brown to brownish grey, 110-280 µm diam, usually immersed in the dense secondary mycelium.
Peridium composed of obscure, irregularly polygonal, ca. 8-20 µm diam cells.
APPENDAGES (ap) few to numerous, mycelioid, simple, rarely irregularly branched, thin-walled, hyaline to coloured, septate, usually shorter than the cleistothecial diameter, located in the lower half of the cleistothecium.
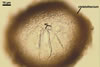 |
ASCI (ac) 6-30 per cleistothecium, stalked, 50-105 x 20-45 µm, (4-)8-spored.
ASCOSPORES (as) ellipsoid to ellipsoid-ovoid, hyaline to faintly coloured, ca. 20-24 x 10-14 µm.
DISTRIBUTION AND HABITAT. Blumeria graminis is a cosmopolite affecting both cereals and ca. 70 species of cultivated and uncultivated grasses (Kochman 1973).
NOTES. The anamorph of B. graminis is Oidium monilioides Link (Holliday 1989).
Blumeria graminis is the only species of the genus Blumeria (Braun 1987). This fungus has originally been described as a member of the genus Erysiphe (Braun 1987). However, the digitate haustoria, secondary mycelium with blister-like hyphae, bulbous swellings of the conidiophores, as well as the structure of the ascocarps (cleistothecia) are unique for this fungus and warranted a separation on generic level (Braun 1987).
Blumeria graminis is an obligate ectoparasite growing on the surface of its hosts and invading only with haustoria (Barnes 1979).
Blumeria graminis is a collective species represented by four formae speciales, i. e., B. graminis f. sp. avenae attacking oat (Avena sativa L.), B. graminis f. sp. hordei occurring on barley (Hordeum vulgare L.), B. graminis f. sp. secalis affecting rye (Secale cereale L.), and B. graminis f. sp. tritici exploiting wheat (Triticum aestivum L.; Holliday 1989). Additionally, each of the formae speciales can produce races (Brooks 1953). These are determined by their behaviour on selected differential varieties of barley, oat, rye, or wheat.
During vegetation, B. graminis mainly spreads by means of oidia of O. monilioides. At the beginning of autumn, the propagules of this fungus may also be early produced ascospores. In spring, the spores infecting other plants are oidia originated from overwintered hyphae and the other ascospores released from cleistothecia. The production of oidia repeats many times.
The conditions favouring infection of plants by both oidia and ascospores of B. graminis are hot and dry weather (Kochman 1973). Infection by B. graminis is optimal at 15-20oC, but can occur over a wide range from 5-30oC (Smith et al. 1988). The infection cycle from conidial germination to sporulation is ca. 7-10 days.
On some varieties of cereals, B. graminis produces brown spots on leaves instead of white powdery patches. The brown spots are dead fragments of leaf tissue, bear only a few inconspicuous hyphae with conidia, and are an indication of resistance (Agrios 1988; Brooks 1953). The necrotic tissue isolates the obligate parasite from its living host on which it depends absolutely for nutrition, and, thereby, results in its death. The faster the host dies after attack, the more resistant to infection the plant is.
REFERENCES
Agrios G. N. 1988. Plant pathology, 3rd edition, Academic Press, INC. San Diego, New York, Berkeley, Boston, London, Sydney, Tokyo, Toronto.
Barnes E. H. 1979. Atlas and manual of plant pathology. Plenum Press. New York and London.
Braun U. 1987. A monograph of the Erysiphales (powdery mildews). Beih. Nova Hedwigia 89, 1-700.
Brooks F. T. 1953. Plant diseases. Geoffrey Cumberlege. Oxford University Press. London, New York, Toronto.
Holliday P. 1989. A dictionary of plant pathology. Cambridge University Press. Cambridge, New York, New Rochelle, Melbourne, Sydney.
Kochman J. 1973. Fitopatologia. PWRiL. Warszawa.
Smith I. M., Dunez J., Lelliott R. A., Phillips D. H., Archer S. A. 1988. European handbook of plant diseases. Blackwell Scientific Publications.