N.C. Schenck & S.M. Sm.
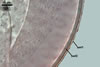
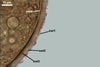
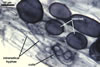
SPORES single in the soil; pale yellow (4A3) to greyish orange (5B5); globose to subglobose; (95-)135(-190) µm diam; sometimes ovoid; 95-110 x 110-160 µm; with one subtending hypha.
Layer 1 mucilagenous, hyaline, (1.5-)2.1(-2.8) µm thick, tightly adherent to layer 2, staining pink (12A4) to purplish red (14A8) in Melzer’s reagent, usually absent or highly deteriorated in mature spores.Layer 2 semiflexible, smooth, hyaline, (2.2-)3.3(-3.9) µm thick, non-reactive in Melzer’s reagent.
Layer 3 laminate, smooth, pale yellow (4A3) to greyish orange (5B5), (2.9-)5.9(-8.1) µm thick, composed of tightly adherent sublayers, <0.5 µm thick.
Layer 4 flexible, smooth, hyaline, (0.4-)0.5(-0.6) µm thick, usually separating from layer 3 in crushed spores, but attached to the inner surface of the laminate layer of a subtending hypha.
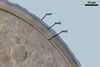
SUBTENDING HYPHA pale yellow (4A3) to grayish orange (5B5); straight to curved; cylindrical or funnel-shaped; (8.3-)12.1(-15.4) µm wide at the spore base.
Wall of subtending hypha pale yellow (4A3) to grayish orange (5B5); (2.7-)3.7(-4.7) µm thick at the spore base; composed of three layers (shwl1-3) continuous with layers 1-3 of the spore wall; layer 1 rarely present in mature spores.Pore closed by spore wall layer 4.