NOTES.
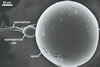
The distinctive properties of S. persica
are its large and sunflower
yellow to apricot yellow
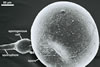
spores ornamented with small warts. The warts
are present in all spores coming from pot cultures.
However, the outermost layer of spores of this fungus
collected from the field frequently is partly smooth.
The warts of these specimens usually are poorly
visible. Uneven distribution of warts or their lack
on a part of the surface of field-collected spores
probably results from the degradative effects of
soil parasites, as Morton (1995) suggested.
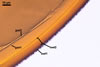
The two-layered wall structure of S. persica
spores is well visible in both specimens collected
from the field and pot cultures. The outer layer
forming the surface of spores propagated in pot
cultures frequently detaches from the laminated
layer 2 of spores stored in a drop of lactic acid
for a few days and, thereby, it is easy to recognize
and characterize. Slight swelling of the outer spore
wall layer in lactic acid also occurs in S.
armeniaca Blaszk. (Blaszkowski, pers. observ.).
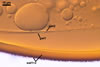
Two thin, hyaline layers constituting the inner
germination wall of S. persica spores usually
are tightly adherent to each other and rarely separate
in even vigorously crushed spores. Hence, they frequently
are visible as a 1-layered structure, especially
in spores coming from the field. Disclosure of the
two layers in the inner wall is relatively easy
when spores slightly crushed with a needle are first
stored in a drop of lactic acid for 12-24 h and
then mounted in PVLG and crushed again.
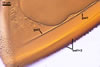
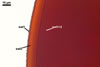
The germination shields of S. persica spores
found by one of the authors of this
website, J. Blaszkowski, are highly ornamented with straight,
Y-shaped, and convoluted ridges. Hence, these properties
resemble those of the germination shield originally
characterized by Koske and Walker (1985), but differ
from those given by Morton (1995). The germination
shield of S. persica spores examined by
Morton (1995) was ornamented with densely distributed
papillate ingrowths and outgrowths. This suggests
that the properties of a germination shield are
variable and, therefore, are a week criterion in
delimitation of species of the genus Scutellospora.
Other species of the genus Scutellospora
forming spores ornamented with warts are S.
coralloidea (Trappe, Gerd. & Ho) Walker
& Koske, S. gregaria (Schenck &
Nicol.) Walker & Sanders, S. heterogama
(Nicol. & Gerd.) Walker & Sanders, and
S. verrucosa (Koske & Walker) Walker &
Sanders. The shape and size of warts are the main
properties separating S. persica (rounded
warts; 0.3-0.5 µm wide x 0.2-0.7 µm
high) from S. coralloidea (flattened warts;
2-12 µm wide x 1-2 µm high) and S.
gregaria (rounded warts; 2-7 µm wide
x 1-2 µm high; Morton 1995; Blaszkowski, pers.
observ.). Scutellospora heterogama produces
smaller spores (100-280 µm diam vs. 270-435
µm diam in S. persica) with larger
warts (1-5 µm high vs. 0.5 µm high in
S. persica) and with two 2-layered inner
walls (vs. one 2-layered wall in S. persica;
Franke and Morton 1994). Spores of S. verrucosa
are lighter-colored [pale-straw to pale yellow-brown
vs. pale to dark copper (Morton
1995) or sunflower
yellow to apricot yellow
in S. persica
(Blaszkowski, pers. observ.)] and have
larger warts (0.5-1 µm wide x 0.5-1 µm
high vs. 0.3-0.5 µm wide x 0.2-0.7µm
high in S. persica; Morton 1995; Blaszkowski,
pers. observ.).
REFERENCES
Bergen
M., Koske R. E. 1984. Vesicular-arbuscular mycorrhizal
fungi from sand dunes of Cape Cod, Massachusetts.
Trans. Br. Mycol. Soc. 83, 157-158.
Blaszkowski
J., Tadych M. 1997. Scutellospora persica
(Glomales, Zygomycetes), an arbuscular mycorrhizal
fungus new to the mycota of Poland. Mycotaxon 65,
379-390.
Friese
C. F., Koske R. E. 1991. The spatial dispersion
of spores of vesicular-arbuscular mycorrhizal fungi
in a sand dune: microscale patterns associated with
the root architecture of American beachgrass. Mycol.
Res. 95, 952-957.
Franke
M., Morton J. B. 1994. Ontogenetic comparisons of
arbuscular mycorrhizal fungi Scutellospora heterogama
and Scutellospora pellucida: revision
of taxonomic character concepts, species descriptions,
and phylogenetic hypotheses. Can. J. Bot. 72, 122-134.
Gemma
J. N., Koske R. E. 1989. Field inoculation of American
beachgrass (Ammophila breviligulata) with
V-A mycorrhizal fungi. J. Environm. Manag. 29, 173-182.
Gemma J. N., Koske R. E., Carreiro M. 1989. Seasonal
dynamics of selected species of VA mycorrhizal fungi
in a sand dune. Mycol. Res. 92, 317-321.
Koske R. E. 1987. Distribution of VA mycorrhizal
fungi along a latitudinal temperature gradient.
Mycologia 79, 55-68.
Koske
R. E., Gemma J. N. 1997. Mycorrhizae and succession
in plantings of beachgrass in sand dunes. Amer.
J. Bot. 84, 118-130.
Koske
R. E., Walker C. 1985. Species of Gigaspora
(Endogonaceae) with roughened outer walls.
Mycologia 77, 702-720.
Morton
J. M. 1995. Taxonomic and phylogenetic divergence
among five Scutellospora species based
on comparative developmental sequences. Mycologia
87, 127-137.