GERMINATION.
Not
observed.
MYCORRHIZAE.
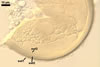
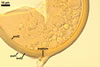
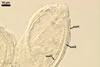
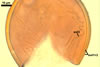
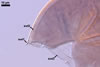
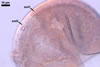
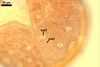
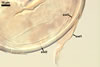
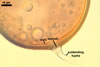
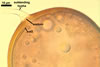
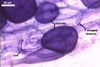
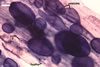
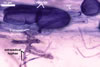
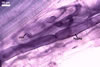
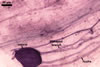
In one-species cultures with the host plant Plantago lanceolata L., mycorrhizae of Gl. walkeri consisted of arbuscules, vesicles, as well as intra- and extraradical hyphae. Arbuscules generally were not numerous and unevenly distributed along root fragments. Vesicles occurred very abundantly and were evenly distributed along root fragments; they were mainly ellipsoid to elongate; 10-50 x 18-215 µm; rarely globose to subglobose; (30-)44(-65) µm diam. Intraradical hyphae were (1.0-)4.5(-7.5) µm wide and grew parallel to the root axis. They were straight or slightly curved, sometimes formed Y- or H-shaped branches and coils. The coils were 20-100 x 27-220 µm. Extraradical hyphae were (2.2-)3.8(-4.7) µm wide. Their abundance varied, depending on the root fragments examined. In 0.1% trypan blue, arbuscules stained violet white (16A2) to greyish violet (16C6), vesicles pastel violet (16B4) to deep violet (16E8), intraradical hyphae violet white (16A2) to campanula violet (17C7), coils greyish violet (16B5-17C5), and extraradical hyphae pastel violet (16A4) to royal purple (16D8).
|
|
|
|
|
|
|
|
|
In roots of P. lanceolata |
PHYLOGENETIC
POSITION. Phylogenetic analysis of LSU placed Gl. walkeri sister to Gl. drummondii and separate from other known Glomus spp. sequences on a branch with high bootstrap support (Figs 1 and 2). In the ITS analysis, reference sequences of Gl. claroideum N.C. Schenck & G.S. Sm., Gl. clarum Nicol. & N.C. Schenck, Gl. etunicatum W.N. Becker & Gerd., and Gl. luteum L.J. Kenn., J.C. Stutz & J.B. Morton, members of Glomus Group B, clustered together, while the sequence of the newly described Gl. walkeri grouped distant (Fig. 3). ITS data again placed Gl. walkeri next to Gl. drummondii and two recently published sequences derived from roots of Taxus. baccata L. (Wubet et al. 2003; Fig. 3). Thus, phylogenetic analysis confirmed the distinctiveness of Gl. walkeri earlier determined based on observations of morphological and biochemical properties of components of its spores.
DISTRIBUTION. The first record of Gl. walkeri comes from a trap culture established from a root-rhizosphere mixture sampled under Oenothera drummondii Hook. colonizing dunes of the Mediterranean Sea adjacent to Tel-Aviv (32º4'N, 34º46'E) in December 2000. One of each trap cultures harbouring spores of this new fungus represented the root zone of Ammophila arenaria (L.) Link growing in the Mediterranean Sea dunes located near Cape Salinas (36o19'N, 3o2'E), Majorca, Spain, in August 2001 and Calambrone (43o35'N, 10o18'E), Italy, in October 2002.
The only species of arbuscular mycorrhizal fungi accompanying Gl. walkeri in Israeli cultures was Gl. intraradices N.C. Schenck & G.S. Sm., and the cultures containing root fragments and soils from Majorca and Calambrone also hosted Gl. coronatum Giovann.
NOTES. Glomus walkeri is distinguished by its single, small and white to pale yellow spores with 3-layered wall, of which the outermost one is a semi-permanent structure staining intensively in Melzer's reagent, and the innermost layer is smooth and easily separates from the structural laminate middle layer.
When observed under a dissecting microscope, spores of Gl. walkeri are similar in appearance to those of Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler, Gl. diaphanum J.B. Morton & C. Walker, Gl. eburneum L.J. Kenn. et al., Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. gibbosum Blaszk., Gl. intraradices, Pacispora franciscana Sieverd. & Oehl, Paraglomus laccatum (Blaszk.) C. Renker, Blaszk. & F. Buscot, and Par. occultum (C. Walker) J.B. Morton & D. Redecker. Another fungus forming spores somewhat resembling those of Gl. walkeri is Gl. minutum Blaszk. et al. However, most spores of Gl. minutum occur in aggregates associated with roots (vs. only singly in the soil in Gl. walkeri) and only its largest spores attain the size of the smallest spores of Gl. walkeri. Additionally, spores of Gl. minutum are hyaline, whereas those of Gl. walkeri are white to pale yellow.
Examination under a compound microscope of spores crushed in PVLG and PVLG mixed with Melzer's reagent readily divides the species listed above into two distinct groups. Only Gl. diaphanum, Gl. fasciculatum, and Gl. gibbosum produce spores with an innermost flexible wall layer of identical properties to that of Gl. walkeri spores (Błaszkowski 1988, 1997, 2003; Kennedy et al. 1999; Morton 2002; Morton and Redecker 2001; Morton and Walker 1984; Oehl and Severding 2004; Pfeiffer, Walker and Bloss1996; Stürmer and Morton 1997; Walker 1982; Walker and Koske 1987). The differences between these species regard the number, as well as the phenotypic and biochemical properties of the other wall layers of their spores. First, although the innermost spore wall layer of Gl. walkeri, Gl. diaphanum, and Gl. fasciculatum is surrounded by only two layers, the outermost wall layer of Gl. diaphanum spores is mucilagenous and short-lived (Błaszkowski 2003; Morton 2002; Morton and Walker 1984; vs. semi-permanent and usually present in mature spores of Gl. walkeri), and the outermost spore wall layer of Gl. fasciculatum is persistent and does not deteriorate with age (Błaszkowski 2003; Walker and Koske 1987; vs. it is more or less degraded in Gl. walkeri at maturity). Second, the middle laminate spore wall layer of Gl. diaphanum is consistently hyaline throughout the life cycle of the fungus (Morton and Walker 1984), whereas that of Gl. walkeri is white to pale yellow. Additionally, this layer in the former fungus is very brittle, which causes the spore to readily break apart or fragment (Błaszkowski, pers. observ.; Morton 2002), a phenomenon not observed in Gl. walkeri. Third, the outermost spore wall layer of Gl. walkeri stains markedly more intensively in Melzer's reagent (up to greyish rose) than that of Gl. diaphanum (light pink; Morton 2002) and Gl. fasciculatum (reddish white; Błaszkowski 2003). In Gl. fasciculatum, its laminate middle layer also reacts in Melzer's reagent (vs. no reaction in Gl. walkeri). Forth, spores of Gl. walkeri occur only singly in the soil, whereas those of Gl. fasciculatum are frequently produced in sporocarps (Błaszkowski 2003; Walker and Koske 1987). Apart from single spores, Gl. diaphanum also produces spores in loose clusters (Morton and Walker 1984). Finally, Gl. diaphanum commonly forms abundant compact clusters of spores in the root cortex of its host plants (Morton and Walker 1984; vs. no intraradical spores in the mycorrhizal roots of Gl. walkeri were found).
In spores of Gl. gibbosum, three layers lie over the innermost layer of their wall (Błaszkowski 1997, 2003; vs. two layers in Gl. walkeri. The laminate layer, similar to that of Gl. walkeri, is coated with a semi-flexible, permanent layer (vs. semi-permanent in Gl. walkeri) associated with an evanescent outermost layer (vs. no such layer in Gl. walkeri); both the layers easily separate from the laminate layer and balloon in lactic acid-based mountants (vs. no ballooning in Gl. walkeri). Other differences between Gl. walkeri and Gl. gibbosum are the lack of reactivity of any spore wall layer of the latter fungus in Melzer's reagent (vs. layer 1 of Gl. walkeri stains in this reagent and the occasional formation of its spores in sporocarps enclosed by a common hyphal mantle (Błaszkowski 1997, 2003; vs. only single spores formed in the soil by Gl. walkeri).
Although Gl. walkeri is morphologically similar to Gl. fasciculatum, a member of Glomus Group A sensu Schüßler et al. (2001), phylogenetic analyses clearly placed it in Glomus Group B. In this group, species forming spores with a flexible innermost wall layer resembling that of Gl. walkeri are Gl. claroideum, Gl. lamellosum, and Gl. luteum. However, all these fungi produce larger and darker-coloured spores that never are white at maturity as Gl. walkeri. Additionally, the spore wall structure of Gl. walkeri and Gl. lamellosum is 3-layered, whereas that of the other species consists of four layers. The spore wall layers of Gl. walkeri and Gl. lamellosum are phenotypically similar. However, compared with Gl. lamellosum, the outermost spore wall layer of Gl. walkeri is much thinner (0.7-1.7 µm vs. 2.2-14.0 µm thick) and stains in Melzer's reagent (vs. no reaction in Gl. lamellosum), and the innermost one is non-reactive in this reagent (vs. it occasionally stains; Błaszkowski 2003; Morton 2002).
Interestingly, phylogenetic analyses placed Gl. diaphanum, a species producing spores highly reminiscent of those of Gl. walkeri, in Glomus Group A. Depending on the mode of phylogenetic analyses, Gl. diaphanum was either closer to the subgroup "a" of Glomus Group A, comprising Gl. caledonium (T.H. Nicolson & Gerd.) Trappe & Gerd., Gl. constrictum Trappe, Gl. mosseae (Nicol. & Gerd.) Gerd. & Trappe, Gl. fragilistratum Skou & I. Jakobsen, Gl. geosporum (Nicol. & Gerd.) C. Walker, and Gl. xanthium Blaszk. et al. (Fig. 2), or fell into the subgroup "b" of Glomus Group A sensu Schwarzott et al. (2001), which is represented by sequence data from the Gl. intraradices/Gl. clarum clade (Fig. 1).
REFERENCES
Błaszkowski J. 1988. Three new vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Poland. Bull. Pol. Ac. Sci. Biol. Sci. 36, 10-12.
Błaszkowski J. 1997. Glomus gibbosum, a new species from Poland. Mycologia 89, 339-345.
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. Address: http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Kennedy L. J., Stutz J. C., Morton J. B. 1999. Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91, 1083-1093.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. Address: West Virginia University. http://invam.caf.wvu.edu.
Morton J. B., Redecker D. 2001. Two families of Glomales, Archaeosporaceae and Paraglomaceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93, 181-195.
Morton J. B., Walker C. 1984. Glomus diaphanum: a new species in the Endogonaceae common to West Virginia. Mycotaxon 21, 431-440.
Oehl F., Sieverding E. 2004. Pacispora, a new vesicular arbuscular mycorrhizal fungal genus in the Glomeromycetes. J. Appl. Bot. 78, 72-82.
Pfeiffer C. M., Walker C., Bloss H. E. 1996. Glomus spurcum: a new endomycorrhizal fungus from Arizona. Mycotaxon 59, 373-382.
Schüßler A., Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105, 1413-1421.
Schwarzott D., Walker C., Schüßler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phylogen. Evol. 21, 190-197.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Walker C. 1982. Species in the Endogonaceae: a new species (Glomus occultum) and a new combination (Glomus geosporum). Mycotaxon 15, 49-61.
Walker C., Koske R. E. 1987. Taxonomic concepts in the Endogonaceae: IV. Glomus fasciculatum redescribed. Mycotaxon 30, 253-262.
Wubet T., Weiß M., Kottke I., Oberwinkler F. 2003. Morphology and molecular diversity of arbuscular mycorrhizal fungi in wild and cultivated yew (Taxus baccata). Can. J. Bot. 81, 255-266.