GERMINATION.
Not
observed.
MYCORRHIZAE.
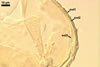
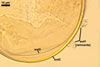
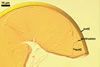
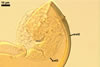
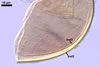
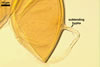
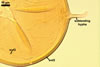
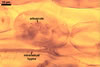
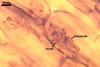
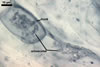
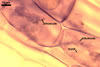
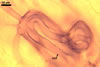
In one-species cultures with Plantago lanceolata L. as the host plant, mycorrhizae of Gl. drummondii comprised arbuscules, vesicles, as well as intra- and extraradical hyphae. Arbuscules were numerous and evenly distributed along the root fragments examined. They consisted of short trunks grown from parent hyphae and numerous branches with very fine tips. Vesicles were not numerous and occurred singly or in aggregates widely dispersed along the roots. They were ellipsoid to prolate; 17.5-90.0 x 30.0-150.0 µm. Intraradical hyphae grew along the root axis, were (2.5-)5.3(-7.8) µm wide, straight or slightly curved, and sometimes formed Y- or H-shaped branches and coils. The coils usually were ellipsoid; 24.0-37.5 x 48.3-90.0 µm; rarely circular; 35.0-40.5 x 35.0-40.5 µm; when seen in a plane view. Extraradical hyphae were (3.0-)4.9(-6.8) µm and occurred in low abundances. In 0.1% trypan blue, arbuscules stained pale violet (16A3) to reddish violet (16C7), vesicles violet white (16A2) to greyish violet (16C4), intraradical hyphae pale violet (16A3) to reddish violet (16B6), coils violet white (15A2) to lilac (16B3), and extraradical hyphae pastel violet (16A4) to greyish violet (16C5).
|
|
|
|
|
|
|
|
|
In roots of P. lanceolata |
|
|
|
In roots of P. lanceolata |
PHYLOGENETIC
POSITION. Phylogenetic analyses of LSU sequences placed Gl. drummondii in Glomus Group B sensu Schüßler et al. (2001; Figs 1 and 2). ITS sequences data placed Gl. drummondii sister to Gl. walkeri Blaszk. & C. Renker (Fig. 3). This clade again is placed sister to species belonging to Glomus Group B (i. e., Gl. claroideum N.C. Schenck & G.S. Sm., Gl. clarum T.H. Nicolson & N.C. Schenck, Gl. etunicatum W.N. Becker & Gerd., and Gl. luteum L.J. Kenn et al.) and two recently published sequences derived from roots of Taxus baccata L. (Wubet et al. 2003). From the original Glomus Group B, reference sequences of Gl. claroideum, Gl. clarum, Gl. etunicatum, and Gl. luteum were included in the ITS analysis; all these sequences clustered together, while sequences of the newly described Gl. drummondii grouped distant, indicating its distinct phylogenetic position (Fig. 2).
DISTRIBUTION.
Glomus drummondii was found for the first time in a trap culture with a root-rhizosphere soil mixture of
Oenothera drummondii Hook. colonizing dunes of the Mediterranean Sea located near Loret de Mar, Costa Brava (41º42'N, 2º51'E), Spain, in August 1999. Subsequently, this fungus was isolated from trap cultures with root fragments and rhizosphere soils of Ammophila arenaria (L.) Link
(4 cultures) of the Baltic Sea dunes adjacent to Jurata (54º38'N, 18º41'E), Jastrzebia Góra (54º5ºN, 18º18'E), and Hel (54º36'N, 18º49'E), as well as of Juncus conglomeratus L. em. Leers
(1 culture) inhabiting a salt march located in Oslonino (54º42'N, 18º28'E), ca. 100 m from the bank of the Puck Bay (all the sites are located in northern Poland and were sampled from 29 August to 5 September of 2000); Zea mays L. (1 culture) cultivated near Faro (37º1'N, 7º56'W), Portugal, in December 2000; O. drummondii (8 cultures) growing along the coast of the Mediterranean Sea extending near El Arenal (39º31'N, 2º45'E) and A. arenaria (9 cultures) colonizing mobile dunes of Cape Salinas (36º19'N, 3º2'E), Majorca, Spain, in August 2001; A. arenaria (7 cultures) growing in sandy dunes of the Mediterranean Sea located near Karabucak-Tuzla (36º43'N, 34º59'E), Turkey, in June 2001; and O. drummondii (1 culture) colonizing sandy areas extending along the bank of the Mediterranean Sea adjacent to Larnaca (34º55'N, 33º38'E), Cyprus, in October 2003.
The occurrence of arbuscular fungi in any field-collected root-rhizosphere soil sample of the plant species listed above was not determined. The only species of arbuscular fungi isolated together with Gl. drummondii from the cultures representing O. drummondii growing in the Loret de Mar dunes and Z. mays cultivated in Portugal was Gl. mosseae (T.H. Nicolson & Gerd.) Gerd. & Trappe. Apart from spores of Gl. drummondii, the trap cultures with Polish soils contained spores of Acaulospora mellea Spain & N.C. Schenck. The fungi co-occurring with Gl. drummondii in Majorca's cultures included A. paulinae Blaszk., Archaeospora trappei (R.N. Ames & Linderman) J.B. Morton & D. Redecker, Diversispora spurca (C.M. Pfeiff., C. Walker & Bloss) C. Walker & Schuessler, Entrophospora infrequens (I.R. Hall) R.N. Ames & R.W. Schneid., Gl. aurantium Blaszk. et al., Gl. constrictum Trappe, Gl. coronatum Giovann., Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. mosseae, Gl. xanthium Blaszk. et al., Pacispora franciscana Oehl & Sieverd., Pac. scintillans (S.L. Rose & Trappe) Sieverd. & Oehl, and Scutellospora calospora (T.H. Nicolson & Gerd.) C. Walker & F.E. Sanders. The cultures from Turkey also contained Gl. arenarium, Gl. constrictum, Gl. coronatum, Gl. deserticola Trappe et al., Gl. intraradices N.C. Schenck & G.S. Sm., Pac. scintillans and an undescribed Glomus 138, and that from Cyprus still hosted Ar. trappei, G. coronatum, and Gl. geosporum (T. H. Nicolson & Gerd.) C. Walker.
NOTES. The morphological characters most distinguishing Gl. drummondii are its small and yellow-coloured spores with their thin, colourless, flexible innermost wall layer intensively staining in Melzer's reagent.
When observed under a dissecting microscope, spores of Gl. drummondii may be confused with small-spored isolates of Gl. arenarium, Gl. claroideum, Gl. etunicatum, Gl. insculptum Blaszk., Gl. lamellosum Dalpé et al., Gl. luteum, and Gl. pustulatum Koske et al. because of their similar appearance and pigmentation.
The first property differentiating Gl. drummondii from all but one of the species listed above is size of their spores. The mean diameter of Gl. drummondii spores overlaps with that of spores of only Gl. insculptum (Błaszkowski et al. 2004). In the other species, only their smallest spores are more or less within the upper diameter range of spores of Gl. drummondii.
Additionally, mature spores of Gl. drummondii, being pastel yellow to maize yellow in colour, are lighter than mature spores of Gl. arenarium (orange to raw umber; Błaszkowski 2003; Błaszkowski et al. 2001), Gl. etunicatum (orange to red brown; Morton 2002), Gl. insculptum (yellowish white to golden yellow; Błaszkowski et al. 2004), Gl. luteum (pale yellow to dark yellow with a brownish tint; Kennedy et al. 1999; Morton 2002), and Gl. pustulatum (deep orange to orange brown; Błaszkowski 1994; Koske et al. 1986).
The highest differences between the fungi compared here reside in phenotypic and biochemical properties, as well as in the number of wall components of their spores. Although Gl. claroideum, Gl. lamellosum, Gl. luteum, and Gl. pustulatum have a thin, hyaline, flexible innermost layer resembling spore wall layer 3 of Gl. drummondii (Błaszkowski 2003; Kennedy et al. 1999; Koske et al. 1986; Stürmer and Morton 1997; Walker and Vestberg 1998), this layer reacts with Melzer's reagent only in Gl. drummondii and Gl. lamellosum. However, the staining reaction is markedly more intensive in the former species [reddish white (7A2) to greyish rose (12B3)] than in the latter fungus [reddish white (7A2) to pastel red (9A4); Błaszkowski 2003]. Additionally, layer 3 of crushed spores of Gl. drummondii easily separates from the laminate layer 2, whereas that of Gl. lamellosum spores usually tightly adheres to this layer and, thereby, is difficult to see. Thus, although this layer in the two species is a component of the spore wall originated from and attached to the upper part of subtending hypha as Stürmer and Morton (1997) found, it is located in groups B and A in the former and the latter species, respectively, following the concept of Walker and Vestberg (1998). Another character readily separating Gl. drummondii and Gl. lamellosum is the relatively thick and long-lived (semi-permanent) outermost layer of the latter fungus (vs. a short-lived and thin layer in the former species).
Spores of Gl. pustulatum also are 3-layered, but the outermost wall layer, forming the spore surface, in this species is a permanent structure (vs. a short-lived one in Gl. drummondii). Moreover, it is ornamented with blister-, cup- or irregularly-shaped processes (Błaszkowski 2003; Koske et al. 1986; vs. smooth or roughened in Gl. drummondii when not sloughed).
Another species producing small spores with a flexible innermost layer easily separating from their laminate layer is Gl. proliferum Dalpe & Declerck (Declerck et al. 2000). However, spores of Gl. drummondii are yellow-coloured, while those of Gl. proliferum are hyaline.
Apart from the biochemical properties of the flexible innermost spore wall layer discussed above, Gl. drummondii, Gl. claroideum, Gl. luteum, and Gl. proliferum also differ in the number and nature of the layers located above the structural, laminate wall layer of their spores. In contrast to the single, sloughing outer layer associated with the laminate wall layer of Gl. drummondii spores, the laminate layer of Gl. claroideum and Gl. luteum is covered with two impermanent layers, of which the outer one stains pink to purplish red in Melzer's reagent (Błaszkowski 2003; Kennedy et al. 1999; Stürmer and Morton 1997; vs. no reaction of layer 1 of Gl. drummondii in this reagent). The laminate layer of Gl. proliferum spores is overlaid with two permanent layers; both are non-reactive in Melzer's reagent (Declerck et al. 2000).
Glomus arenarium and Gl. etunicatum diverge from Gl. drummondii mainly because of the lack of the flexible innermost layer of the last fungus.
The close relationship of Gl. drummondii with Gl. claroideum, Gl. lamellosum, and Gl. luteum, members of Glomus Group B sensu Schüßler et al. (2001), found based on comparison of their morphological and biochemical characters, was also mirrored in the phylogenetic analyses performed (Figs 1 and 2). These placed Gl. drummondii in Glomus Group B and, thereby, in the vicinity of the three obove mentioned Glomus spp.
REFERENCES
Błaszkowski J. 1994. Polish Glomales 11. Glomus pustulatum. Mycorrhiza 4, 201-207.
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone, and Complexipes species deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. Address: http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Błaszkowski J., Adamska I., Czerniawska B. 2004. Glomus insculptum, a new arbuscular mycorrhizal species from Poland. Mycotaxon 89, 225-234.
Błaszkowski J., Tadych M., Madej T. 2001. Glomus arenarium, a new species in Glomales (Zygomycetes). Acta Soc. Bot. Pol. 70, 97-101.
Declerck S., Cranenbrouck S., Dalpé Y., Séguin S., Grandmougin-Ferjani A., Fontaine J., Sancholle M. 2000. Glomus proliferum sp. nov.: a description based on morphological, biochemical, molecular and monoxenic cultivation data. Mycologia 92, 1178-1187.
Kennedy L. J., Stutz J. C., Morton J. B. 1999. Glomus eburneum and G. luteum, two new species of arbuscular mycorrhizal fungi, with emendation of G. spurcum. Mycologia 91, 1083-1093.
Koske R. E., Friese C., Walker C., Dalpé Y. 1986. Glomus pustulatum: A new species in the Endogonaceae. Mycotaxon 26, 143-149.
Morton J. B. 2002. International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi. Address: West Virginia University. http://invam.caf.wvu.edu.
Schüßler A., Schwarzott D., Walker C. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol. Res. 105, 1413-1421.
Stürmer S. L., Morton J. B. 1997. Developmental patterns defining morphological characters in spores of four species in Glomus. Mycologia 89, 72-81.
Walker C., Vestberg M. 1998. Synonymy amongst the arbuscular mycorrhizal fungi: Glomus claroideum, G. maculosum, G. multisubstensum and G. fistulosum. Annal. Bot. 82, 601-624.
Wubet T., Weiß M., Kottke I., Oberwinkler F. 2003. Morphology and molecular diversity of arbuscular mycorrhizal fungi in wild and cultivated yew (Taxus baccata). Can. J. Bot. 81, 255-266.